Immune to Cancer: The CRI Blog
-

FDA Approves Nivolumab (Opdivo) for Advanced Hodgkin Lymphoma Patients
Nivolumab becomes first checkpoint immunotherapy approved for lymphoma.
-
FDA Regulatory Definitions
You heard that a drug in clinical trials was Fast Tracked. Or you heard that a treatment…
-

FDA Approval Expands Immunotherapy Options for Advanced Melanoma
Immunotherapy combinations continue to shine against melanoma.
-

Immunotherapy Approvals in 2015
In 2015, the FDA approved more immunotherapies than in any prior year.
-
Second Antibody FDA-Approved Against Multiple Myeloma
Elotuzumab (Empliciti) is the second antibody for myeloma approved in just two weeks.
-
Nivolumab Becomes First FDA-Approved Checkpoint Blockade for Kidney Cancer
New hope now exists for patients with advanced kidney cancer.
-
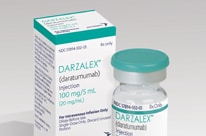
First Monoclonal Antibody for Multiple Myeloma Receives FDA Approval
Daratumumab (Darzalex®) is the first approved immunotherapy that targets CD38—a protein found in most multiple myeloma cells.
-
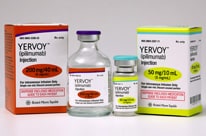
FDA Approves Immunotherapy to Help Prevent Relapse of Melanoma
The immunotherapy drug ipilimumab (Yervoy®) can now be used to help prevent relapse after surgery.
-
FDA Approves First in a New Class of Immunotherapies
Amgen’s oncolytic virus immunotherapy, T-VEC, gets the green light from the FDA.