Immune to Cancer: The CRI Blog
-
First Checkpoint Immunotherapy Approved for Advanced Cervical Cancer
Pembrolizumab, a PD-1-targeting immunotherapy, was approved for recurrent advanced cervical cancer that expresses PD-L1.
-
Second CAR T Cell Immunotherapy Approved for Patients with Lymphoma
A second CAR T cell immunotherapy targeting the CD19 receptor was approved by the FDA for patients…
-

AACR18 Recap: Immunotherapy in the Spotlight
Immunotherapy took center stage and dominated the headlines at AACR18.
-

Checkpoint Immunotherapy Combination Approved for First-line Treatment of Advanced Kidney Cancer
The combination of checkpoint immunotherapies against PD-1 and CTLA-4 significantly improved survival and response rates compared to…
-
Immunotherapy is Here to Stay: Looking Back at this Year’s Breakthroughs
Important immunotherapy breakthroughs benefited patients immensely in 2017 and paved the way for even more advances in…
-

Recent FDA Announcements Herald Advances in Personalized Approaches Against Cancer
Two tumor-profiling tests recently authorized by the FDA enable doctors to tailor their treatments more effectively to…
-
CAR T Cell Immunotherapy Approved for Adult Non-Hodgkin Lymphoma Patients
FDA approves new immunotherapy–Yescarta (axicabtagene ciloleucel)–for adult patients with relapsed or refractory non-Hodgkin large B cell lymphoma.
-

Checkpoint Immunotherapy Approved for Patients with Stomach, Gastroesophageal, and Liver Cancer
Previously treated patients with these cancer types can now receive anti-PD-1 immunotherapy; nivolumab for HCC and pembrolizumab…
-
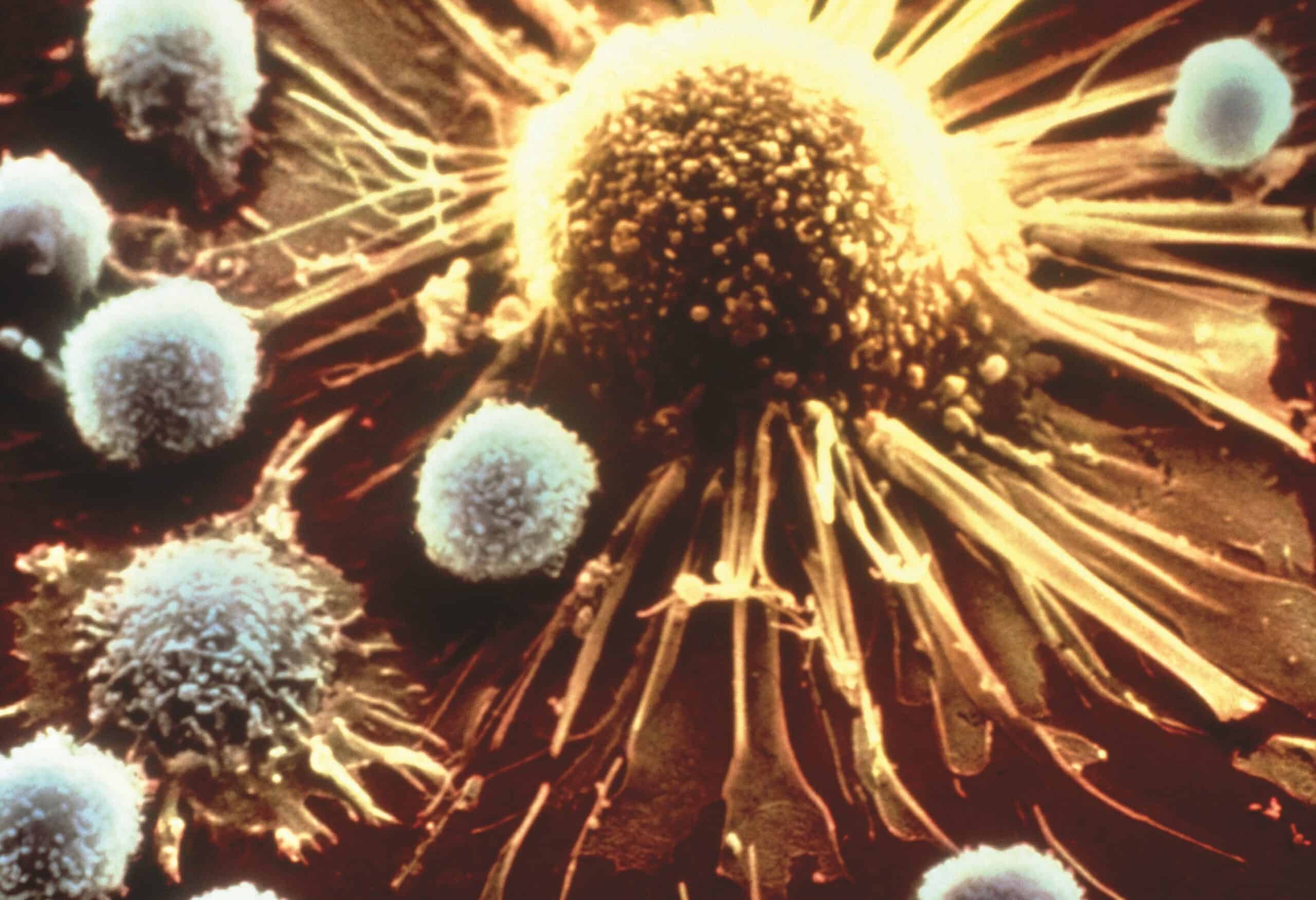
FDA Approves First-In-Class CAR T Cell Immunotherapy for Leukemia
Children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL) can now receive the CD19-targeting CAR T…