Immune to Cancer: The CRI Blog
-
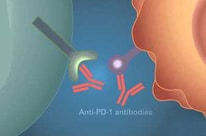
FDA Approves Expanded Use of Nivolumab for Lung Cancer
Nivolumab, a PD-1 immunotherapy, is now approved as second-line therapy for the most common type of lung…
-
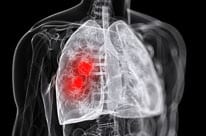
FDA Approves PD-1 Immunotherapy for Most Common Type of Lung Cancer
Patients with non-small cell lung cancer may now receive a PD-1 immunotherapy as second-line treatment after chemotherapy.
-
In a First, FDA Approves Immunotherapy Combination for Advanced Melanoma
The approval of a combination of two immunotherapy drugs for melanoma represents a treatment milestone.
-
European Regulators Approve Pembrolizumab (Keytruda) as First-line Treatment of Advanced Melanoma
The decision makes pembrolizumab the second PD-1-blocking drug to receive approval in Europe.
-

What Cancer Patients Need to Know About the Opdivo Approval
Now that Opdivo (nivolumab) has been FDA approved for two cancer types, many patients are wondering: what’s…
-

Big News: FDA Approves Opdivo (Nivolumab) for Lung Cancer
The PD-1 checkpoint inhibitor Opdivo® (nivolumab) is a new treatment option for patients with non-small cell lung…
-
New Cancer Immunotherapy Opdivo Approved by FDA
Just hours ago, the FDA approved Bristol-Myers Squibb's PD-1 drug for melanoma.
-
FDA Approves New Immunotherapy for Leukemia
Amgen’s Blincyto was approved by the FDA, making it the first approved bispecific T cell engaging (BiTE)…
-
FDA Approves New Cancer Immunotherapy
Merck's new immunotherapy, Keytruda (pembrolizumab), was approved today by the FDA, making it the first PD-1 inhibitor…