Immune to Cancer: The CRI Blog
-

AACR18 Day 2 Update: What’s Next for Cancer Immunotherapy?
CRI scientists kicked off the opening plenary session at AACR18 and discussed several promising avenues of immunotherapy…
-

AACR18: Driving Innovative Cancer Science to Patient Care
This year’s annual AACR meeting boasts an impressive lineup of presentations highlighting both clinical and preclinical immunotherapy…
-

Recent FDA Announcements Herald Advances in Personalized Approaches Against Cancer
Two tumor-profiling tests recently authorized by the FDA enable doctors to tailor their treatments more effectively to…
-
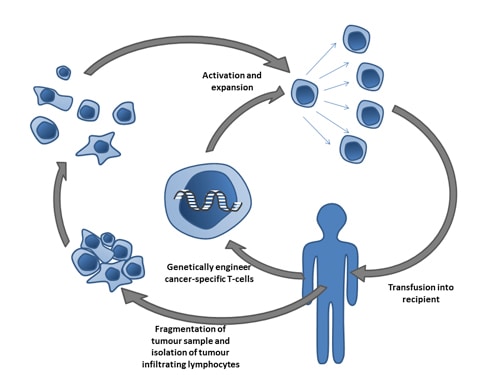
CICON17 Day 2 Recap: Biomarkers, Novel Agents, and Adoptive Cell Therapies
Day 2 of CICON17 offered a focused exploration of the latest discoveries and data from scientific studies…
-

Highlights of the 2nd Annual Rational Combinations 360° Conference
Biomarkers will be crucial to taking advantage of combination immunotherapy’s full potential
-
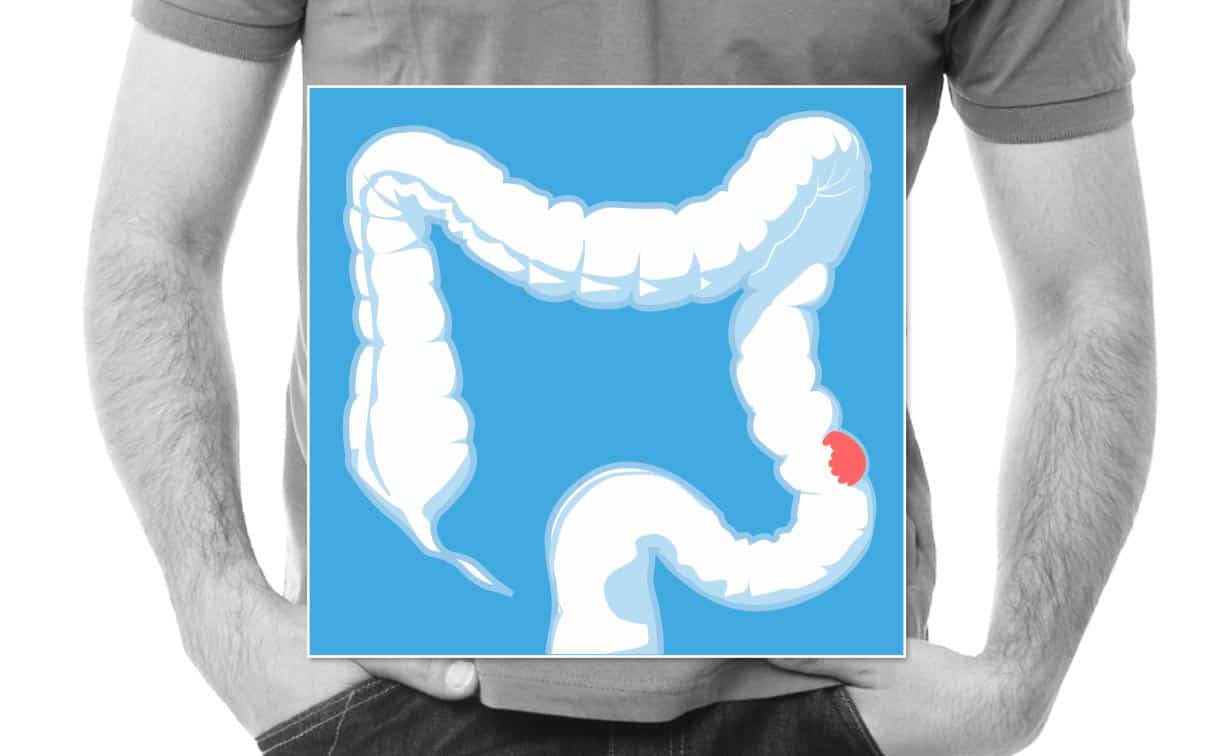
Nivolumab Approved for Patients with Chemotherapy-Resistant, Metastatic, MSI-H Colorectal Cancer
Nivolumab becomes second checkpoint immunotherapy approved for colorectal cancer
-

Immunotherapy Makes History with Latest Cancer Approval
The FDA’s biomarker-based approval of Keytruda was the first of its kind
-

ASCO 2016 Update: Biomarker-Based Strategies
Molecular clues may help predict patient treatment and outcomes.