30 Facts About Immunotherapy
Discover different aspects of the science, history, development, and impact of cancer immunotherapy with 30 facts about immunotherapy. For each day in June, learn about the science of the immune system and how it works, how immunotherapy is able to take advantage of the immune system’s power to fight cancer and immunotherapy’s potential benefits for cancer patients.
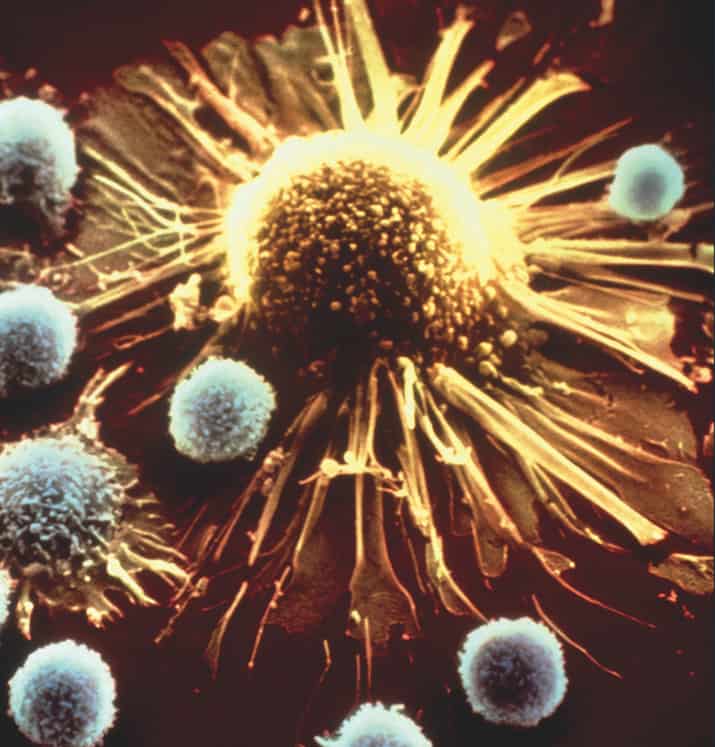
FACT #1
Immunotherapy can empower your immune system against cancer
Read more
The immune system is naturally equipped to protect us against cancer. Cytotoxic T lymphocytes—otherwise known as killer T cells—are especially effective at targeting tumors. However, cancers sometimes figure out how to outsmart the immune system and protect themselves.
Immunotherapy aims to reverse that situation.
Over the next thirty days, we’ll be highlighting a wide scope of immune-based approaches that are already improving outcomes for patients. Many of these treatments work by either directly or indirectly enhancing the activity of T cells (the blue/white cells pictured aboved).
Much of what we know about the immune system and its relationship to cancer was discovered by scientists affiliated with the Cancer Research Institute (CRI), which since 1953 has served as the world’s leading (and for several decades only) nonprofit organization dedicated exclusively to transforming cancer patient care by advancing immunotherapy and the science behind it.

FACT #2
1891: Dr. William B. Coley—CRI’s “grandfather”—uses first immunotherapy to save a patient with inoperable cancer.
Read more
In the late 1800s, surgery was really the only decent option available to cancer patients. This frustrated Dr. William B. Coley, especially after he was unable to save a patient named Bessie Dashiell, who was a close friend of John D. Rockefeller Jr.
So, in 1891, inspired by a case he’d read about, Coley decided to try something new. He used bacteria to purposely infect a New York City man named Zola, who had many advanced tumors, including one in his throat that prevented him from eating.
Amazingly, it worked, and soon after, Zola resumed his normal life.
For many reasons, including the advent of radiation and then chemotherapy, Coley’s approach was largely dismissed during his lifetime. Immunotherapy would not be forgotten though, and in 1953, Coley’s daughter, Helen, with the help of a $2,000 grant from John D. Rockefeller Jr.’s son Nelson, launched the Cancer Research Institute (CRI).
To honor and commemorate the “Father of Immunotherapy”, CRI bestows an annual William B. Coley Award that recognizes scientists whose work has deepened our understanding of the immune system’s cancer-fighting capabilities and advanced the development of effective immunotherapies for patients. You can learn more about Dr. Coley’s story and the history of the Cancer Research Institute here.
FACT #3
Immunotherapy was named ASCO’s “Advance of the Year” each of the last two years.
Read more
In February, for the second year in a row, the American Society of Clinical Oncology (ASCO) named immunotherapy the “Advance of the Year.”
It’s clear now that immunotherapy can provide long-term benefits to sizable subsets of patients with diverse types of cancer, and new clinical breakthroughs are happening all the time. Thus far in 2017, there have been eight immunotherapy approvals, with two immunotherapies (durvalumab and avelumab) gaining approval for the first time. These clinical breakthroughs were made possible in part by decades of discoveries made by Cancer Research Institute (CRI) scientists.
One of the most important figures who helped advance immunotherapy (and the science that supports it) to this point was Dr. Lloyd J. Old, who actually worked with Dr. William B. Coley’s daughter, Helen, at CRI.
Now known as the “Father of Modern Tumor Immunology,” Dr. Old directed CRI’s scientific and medical efforts for 40 years (1971-2011), during which time he made major discoveries about the immune system and cancer, and helped establish the scientific foundation upon which today’s immunotherapies were developed.
FACT #4
There are currently five different classes of immunotherapies available for patients, for over 20 cancer types.
Read more
As of June 2017, the U.S. Food and Drug Administration (FDA) has approved 32 different immunotherapies for patients with cancers, including but not limited to melanoma, lung cancer, bladder cancer, kidney cancer, lymphoma, leukemia, and prostate cancer. These immunotherapies enhance the cancer-fighting activity of the immune system in a variety of ways, which can be roughly divided into the five following classes:
Cell-based immunotherapies physically supplement patients’ immune systems with immune cells. These include bone marrow transplants and newer, more sophisticated cell transplants, such as CAR T cells.
Immunomodulators can act directly on immune cells to promote anti-cancer activity. The first immunomodulator (the cytokine IFN-α) was approved by the FDA in 1986 for leukemia (after CRI provided the seed funding for the first significant trial in humans), while the first checkpoint inhibitor (the anti-CTLA-4 ipilimumab) was approved in 2011 for advanced melanoma.
Vaccines help educate or arouse the immune system against a potential threat. In 1990, a tuberculosis vaccine called BCG (bacillus Calmette-Guerin), became the first to be FDA-approved in the United States, for the treatment of bladder cancer. The vaccine Sipuleucel-T was approved for prostate cancer patients in 2010.
Antibody-based targeted therapies can target cancer cells directly, or other cells/proteins that help support tumor survival. The first antibody (the anti-CD20 rituximab) was approved in 1997 for lymphoma. There are now over a dozen antibody-based immunotherapies approved for various cancers, including modified versions with anti-cancer drugs attached.
Oncolytic viruses can be modified to infect cancer cells and cause them to burst (think of dynamite!), which attracts the attention of the immune system. The oncolytic virus T-Vec was approved for patients with advanced melanoma in 2015.
CRI scientists made major discoveries and crucial contributions that enabled the development of (or improved) nearly all of these immunotherapies, which we will discuss in more depth throughout the rest of this campaign.
FACT #5
Over 100 billion immune cells are produced every day in a person’s bone marrow.
Read more
All of our blood cells—both red (oxygen-transporting) and white (immune/disease-fighting)—are generated from stem cells that reside inside the marrow of our bones. All in all, these stem cells give rise to over a dozen different subsets of immune cells. CRI scientists discovered several of these immune cell types, including Natural Killer (NK) cells (Georg Klein, M.D, D.Sc.) and regulatory T cells (Shimon Sakaguchi, MD, PhD).
Immunotherapies such as bone marrow transplants (also called stem cell transplants) have long been used to treat leukemia and restore patients with healthy immune systems.
However, there are still significant issues associated with these transplants. In addition to the toxicity of pre-transplant chemotherapy, transplants aren’t always effective. And even when they do work, patients still often have to take immunosuppressive drugs that can leave them vulnerable to infection and future cancer relapse.
The following CRI-funded scientists are researching ways to overcome these issues and improve both the safety and effectiveness of bone marrow transplants for cancer patients.

FACT #6
The two branches of our immune system co-operate to provide general defense and customized responses against cancer.
Read more
The immune system has both innate and adaptive components that complement each other. Innate cells can quickly respond to common threats (infections, cancer, etc.) through their ability to recognize to potential signs of danger such as foreign molecules or genetic material in improper places.
These innate responses can help get things under control initially, but they aren’t great at adapting to threats (like cancer) that evolve over time. Fortunately, once the innate cells sound the alarm, they also stimulate adaptive immune cells (such as T cells) to carry out incredibly precise responses against tumors.
Three CRI scientists—Lloyd J. Old, MD, Robert D. Schreiber, PhD, and Mark J. Smyth, PhD, FAHMS—provided the first definitive evidence of this natural protective ability when they showed that mice without adaptive abilities developed cancer much more often than normal mice. (For obvious reasons, this experiment has not been performed in humans.)
In addition to many others investigating adaptive immunity, the following CRI-funded scientists are currently exploring how innate immune cells and pathways could be used to improve the effectiveness of anti-tumor immune responses:

FACT #7
Some immune cells attack cancer directly, while others help coordinate the response.
Read more
Just like any well-trained force, our immune cells divide the labor, too.
Some, like T cells, attack tumors directly. Others, such as dendritic cells (DCs), orchestrate attacks from behind the scenes. By gathering intelligence about a tumor’s markers (antigens), DCs can then educate other immune what the tumor “looks like.”
This “education,” which often takes place in the lymph nodes, occurs through a system of surface receptors called MHC (major histocompatibility complex) that present tumor antigens. The MHC/antigen system is what gives the adaptive immune system the ability to recognize and respond to threats, including cancer and infection. (The MHC2 molecule was discovered by Peter Cresswell, PhD , while Emil R. Unanue, MD, and Rolf M. Zinkernagel, MD, PhD, also made major contributions to our understanding of MHC activity.)
Because of this ability to coordinate anti-tumor responses, DCs—which were discovered by Ralph Steinmann, MD—have been incorporated into several immunotherapy approaches, including Sipuleucel-T, an FDA-approved prostate cancer vaccine that uses a patient’s own DCs.
The following CRI-funded scientists are investigating the factors that influence the MHC/antigen system and adaptive immune responses, as well as evaluating novel DC-based immunotherapy approaches for patients:
FACT #8
Macrophages—literally the “big eaters” of the immune system—can devour cancer cells.
Read more
Macrophages (Greek for “big eaters”) are innate immune cells that are capable of physically ingesting damaged or diseased cells through a behavior called phagocytosis (cell-devouring process).
However, sometimes tumors are able to protect themselves by manipulating macrophages—hijacking them into helping cancer spread. Fortunately, immunotherapies have been developed to target macrophages and make sure they stay on the right side of the fight against cancer.
For instance, cancer cells can tell macrophages “don’t eat me!” by expressing the CD47 receptor on their surfaces. To overcome that, immunotherapies that block CD47 have been developed and are currently being evaluated in clinical trials. Macrophages can also aid tumor growth and metastasis, the process in which a tumor spreads to other organs, and doctors investigating effective approaches to target these behaviors, too.
The following CRI-funded scientists are exploring the complex relationship between macrophages and cancer, as well as identifying and testing promising new targets to enhance their anti-cancer activity with immunotherapy:
FACT #9
B cells can create trillions of unique antibodies that provide precision targeting.
Read more
As we mentioned earlier, the immune system uses the MHC/antigen system to target tumors. While both B and T cells are activated through the MHC/antigen system, once activated they go after tumors in distinct ways.
Whereas T cells physically attach to and attack cancer cells, B cells target them indirectly through the production of molecules called antibodies (that can bind to tumor antigens). The specific, powerful binding of these antibodies can then disrupt crucial growth signaling pathways (by blocking their surface receptors) as well as mark the cancer cells for destruction by other immune cells.
B cells also have proteins called RAGs (recombination-activating genes) that enable them to rearrange the components of antibodies and customize their shapes. With the help of these RAGs, which were first discovered by Susumu Tonegawa, PhD, it’s been estimated that B cells have the potential to synthesize over 1012 (one trillion!) distinct antibodies.
Unfortunately, abnormal RAG activity can also cause cancer-promoting mutations in B cells, and is believed to contribute to the development of lymphoma. This connection was discovered by Frederick W. Alt, PhD and Klaus Rajewsky, MD
Outside of B cells, scientists now also have the ability—thanks in part to Jeffrey V. Ravetch, MD, PhD— to produce synthetic, customized antibodies for cancer immunotherapy. Over a dozen of these have already been approved by the FDA, for many diverse cancer types. Examples include anti-CD20 antibodies for leukemia and lymphoma and anti-HER2 antibodies for breast cancer.
The following CRI-funded scientists are working to better understand the factors that govern B cell activity and antibody production, with a focus on developing strategies to prevent and/or treat cancer in the clinic:

FACT #10
Viruses can cause cancer.
Read more
an trigger genetic mutations that lead to cancer. The first virus to cause cancer in humans wasn’t identified until the 1960s, when Lloyd J. Old, MD, and Georg Klein, MD, D.Sc., helped characterize the link between Epstein-Barr virus (EBV) and certain cancers (nasopharyngeal cancer, certain types of lymphomas, and stomach cancer).
Many other viruses are now known to contribute to cancer in humans, including the human papillomavirus or HPV (associated with cervical cancer and head and neck cancer) and hepatitis B virus or HBV (associated with liver cancer). A connection between HIV (human immunodeficiency virus) and Kaposi’s sarcoma was identified by Bijan Safai, MD, D.Sc., who also aided in HIV’s initial identification. Other viruses associated with cancer include human T-lymphotropic virus, hepatitis C (HCV) virus, and Merkel cell polyomavirus.
Vaccines can help prevent cancers caused by these viruses. Vaccines do not cause cancer.
LEARN MORE ABOUT THE CAUSES OF CANCER
How do vaccines prevent cancer?
Viruses work—and contribute to cancer—by sneakily inserting their genes into our DNA, which can disrupt and de-regulate the genetic activity of our cells and cause them to grow in inappropriate ways. Fortunately, vaccines have been developed that can prepare our immune systems against certain infections and therefore help protect us against the cancers that they can cause. Already, approved vaccines are available against HBV and some strains of HPV, which can help protect against liver cancer and several other types of cancer, respectively. In addition to preventive vaccines, scientists and physicians are researching therapeutic vaccines that can be used to treat cancer.
LEARN MORE ABOUT CANCER VACCINES
Harnessing the power of the immune system.
Understanding the power of the immune system has allowed us to create vaccines that prevent cancer and other cancer treatments that use the body’s own immune system to control and eliminate cancerous cells, known as immunotherapy. Your support of the Cancer Research Institute directly impacts cancer immunotherapy research and the development of vaccines to treat and prevent cancer. You can donate today to CRI to support more breakthroughs and save more lives, or explore other ways to join our cause.
With your support, the Cancer Research Institute can, in turn, fund doctors and scientists leading immunology research and working to develop immunotherapy as a cure for all cancers. The following CRI-funded scientists are working to better understand how our immune system works to combat viruses, in addition to developing strategies that can improve the effectiveness of vaccination against certain cancer-causing viruses:
- Using a viral model called LCMV, Mohamed Abdel Hakeem, PhD, is exploring epigenetic patterns associated with immune cell exhaustion that might suggest ways to prevent and reverse it.
- Sadeem Ahmad, PhD, is investigating which RNAs turn on MDA5, an immune receptor that recognizes and responds to viral infections and tumor cells.
- Jonathan M. Clingan, PhD, is investigating how the OAS-RNase L pathway, which may sense a cancer-causing virus infection, enhances or dampens the immune response.
- William H. Hudson, PhD, is looking at how long non-coding RNAs regulate gene expression during T cell development and during responses to viral infection.
- Tuo Li, PhD, identified a switch on the protein that produces cGAMP, a DNA sensing system that can alert the immune system and would be beneficial in a cancer vaccine.
Discover different aspects of the science, history, development, and impact of cancer immunotherapy with 30 facts about immunotherapy, presented as part of our fifth annual Cancer Immunotherapy Month.
Stay up-to-date with the latest in cancer immunotherapy news by signing up for our e-newsletter.

FACT #11
Mutations make tumors “stand out” to the immune system.
Read more
Previously, we discussed how viruses can cause cancer by de-regulating the normal activity of our genes. In addition to viruses, our genes can also become destabilized as a result of random mutations. These DNA mutations can be caused by environmental factors such as ultraviolet (UV) light and cigarette smoke, and can also happen as a result of accidental mistakes during cell division. These mutations—some of which produce abnormal, mutated proteins—can promote the growth and survival of cancer in a number of ways.
Fortunately, these mutated tumor proteins also distinguish cancer cells from healthy cells (because of their “foreign” appearance) and allow immune cells to recognize and respond to tumors. Immunotherapies have also been designed to take advantage of this fact. In fact, patients whose tumors have very high levels of mutations are much more likely to respond to a type of immunotherapy called checkpoint inhibition (more on this later).
The following CRI-funded scientists are exploring how exactly the immune system recognizes these mutations and generates responses against them, as well as how novel immunotherapy approaches might be able to enhance outcomes in patients:

FACT #12
Vaccines can also help people who already have cancer.
Read more
Most people, when they hear the word vaccine, think about preventing diseases rather than treating diseases that people already have. In addition to vaccines that can help prevent cancer, more recently, vaccines have been developed that can help treat patients who already have cancer. These work by helping the immune system learn what cancer “looks like.”
The first vaccine to be approved by the FDA was BCG (bacillus Calmette-Guerin)—which is actually a live, but weakened bacteria that was first used as a tuberculosis vaccine—for early-stage bladder cancer in 1990. (CRI’s Lloyd J. Old, MD, was the first to show that BCG was effective against tumors in mice.)
More recently, the vaccine sipuleucel-T was approved for prostate cancer in 2010. Unlike BCG, which incidentally stimulates the immune system against cancer, sipuleucel-T is purposely designed to stimulate an immune response against cancer. The treatment involves first removing dendritic cells (DCs) from cancer patients and exposing them to a prostate cancer-specific antigen (known as prostatic acid phosphatase, or PAP), before putting them back into patients where they can hunt down and eliminate tumor cells.
Another newer approach pioneered by Kunle Odunsi, MD, PhD, (pictured) has used a vaccine that targets the NY-ESO-1 protein and is associated with improved survival in patients with ovarian cancer that expresses NY-ESO-1.
Moving forward, scientists are focused on developing more personalized vaccines for patients, and these vaccines can be designed to target each patient’s individual mutations. To that end, the Cancer Research Institute, in collaboration with the Parker Institute for Cancer Immunotherapy, recently launched an initiative called TESLA that aims to refine an approach for making personalized vaccines for individual patients.
In addition to TESLA, the following CRI-funded scientists are also working on ways to improve the effectiveness of vaccines for cancer patients:
FACT #13
The immune system’s memory allows it to “remember” cancer and provide long-term protection.
Read more
Previously, we discussed how our adaptive immune system can create customized responses that can target tumors with incredible precision. While an adaptive immune response will ideally eliminate the tumor cells once and for all, sometimes cancer cells use their tricks to escape and hide from the immune system. These can remain dormant and cause patient relapse in the future.
Fortunately, once an adaptive immune response has been designed against a tumor, the immune system can “remember” the cancer’s identity—often for years—and has the ability to quickly launch a follow-up response should the tumor return. These memory T cells play a crucial role in providing long-term protection to cancer patients and therefore are a big focus of research efforts to improve immunotherapy strategies.
The following CRI-funded scientists are working to better understand immune memory and its applications in immunotherapy:
FACT #14
Cancer can exhaust immune cells. Checkpoint immunotherapies can wake them back up.
Read more
While the immune system naturally has the ability to eradicate tumors, in some circumstances they can instead be prevented from doing so. Immune checkpoint pathways are one of the mechanisms that can suppress and/or “exhaust” anti-cancer immune cells, which makes them tolerant of cancer. To address that, scientists have developed checkpoint inhibitor immunotherapies that block the activity of those pathways, re-energizing these immune cells and restoring their cancer-fighting abilities. These work especially well against heavily mutated tumors that the immune system has already recognized and mounted an initial response against.
CRI Scientific Advisory Council Director James P. Allison, PhD, was the first to identify an immune checkpoint pathway, the CTLA-4 receptor. His discovery then led to the development of ipilimumab, an anti-CTLA-4 checkpoint immunotherapy, which was first approved by the FDA in 2011 for melanoma. Since then, several other checkpoint pathways—most notably the PD-1/PD-L1 pathway—have been identified and immunotherapies have been developed to target them. Currently, there are six FDA-approved checkpoint immunotherapies. Two of them, ipilimumab and nivolumab (an anti-PD-1 checkpoint immunotherapy) are approved in combination for the treatment of melanoma, while pembrolizumab (anti-PD-1) is approved as a first-line option for patients with advanced lung cancer and atezolizumab (anti-PD-L1) is approved as a first-line option for patients with advanced bladder cancer who are ineligible for chemotherapy.
The following CRI-funded scientists are exploring how to improve the effectiveness of current checkpoint immunotherapies as well as identifying and evaluating the therapeutic potential of new checkpoints:
- Lei Zheng, MD, PhD
- Mohamed Abdel Hakeem, PhD
- Sadeem Ahmad, PhD
- Scott J. Antonia, MD, PhD, Georgina V. Long, MD, PhD, Richard A. Scolyer, MD, Mark John Smyth, PhD, FAHMS, and John Stagg, PhD
- Matteo Bellone, MD
- Nina Bhardwaj, MD, PhD, and Sacha Gnjatic, PhD
- Adam N. R. Cartwright, PhD
- Michael A. Curran, PhD
- Rony Dahan, PhD
- Susan M. Kaech, PhD
- Amy K. Kim, MD
- John M. Kirkwood, MD, and Hassane M. Zarour, MD
- Stephen Mok, PhD
- Drew M. Pardoll, MD, PhD, Antoni Ribas, MD, PhD, and Cassian Yee, MD
- David M. Owens, PhD
- Christophe Pedros, PhD
- Stephen P. Schoenberger, PhD
- Chong Sun, PhD
- Haidong Tang, PhD
- Ryan M. Teague, PhD
- Derek Alan Wainwright, PhD
FACT #15
Biomarkers can help personalize immunotherapy to fit individual patients.
Read more
Traditionally, patients have been treated in a one-size-fits-all manner, usually based on the location, rather than the specific characteristics, of their tumors. Now, with the rise of immunotherapy, doctors have increasingly focused on using biomarkers—which can be defined as measurable factors that shed light on a patient’s or tumor’s biology—to predict which types of immunotherapy approaches will work best for which patients.
With checkpoint inhibitor immunotherapies for example, patients whose biomarkers indicate that their immune system has already recognized and responded to the tumor are much more likely to benefit from this type of immunotherapy, which gives their immune cells an extra boost that can help them finish the job. Similarly, if a patient’s tumor cells express a certain protein (such as NY-ESO-1) that healthy cells do not normally express, then this lets doctors know that an immunotherapy approach that targets NY-ESO-1 could benefit that patient.
Recently there has been great progress in this area. CRI researchers showed that the biomarker Bim could help doctors predict which patients are most likely to benefit from anti-PD-1 checkpoint immunotherapy (as well as monitor them during treatment to gauge its effectiveness). Furthermore, the FDA’s historic approval of pembrolizumab (anti-PD-1 checkpoint immunotherapy) in May was the first time they’d ever approved a cancer treatment for patients based on a biomarker (in this case microsatellite instability, or MSI) rather than the tumor’s origin.
Many different types of potential biomarkers have been discovered, and the following CRI-funded scientists are working to identify the ones that hold the most potential for improving immunotherapy outcomes for patients:
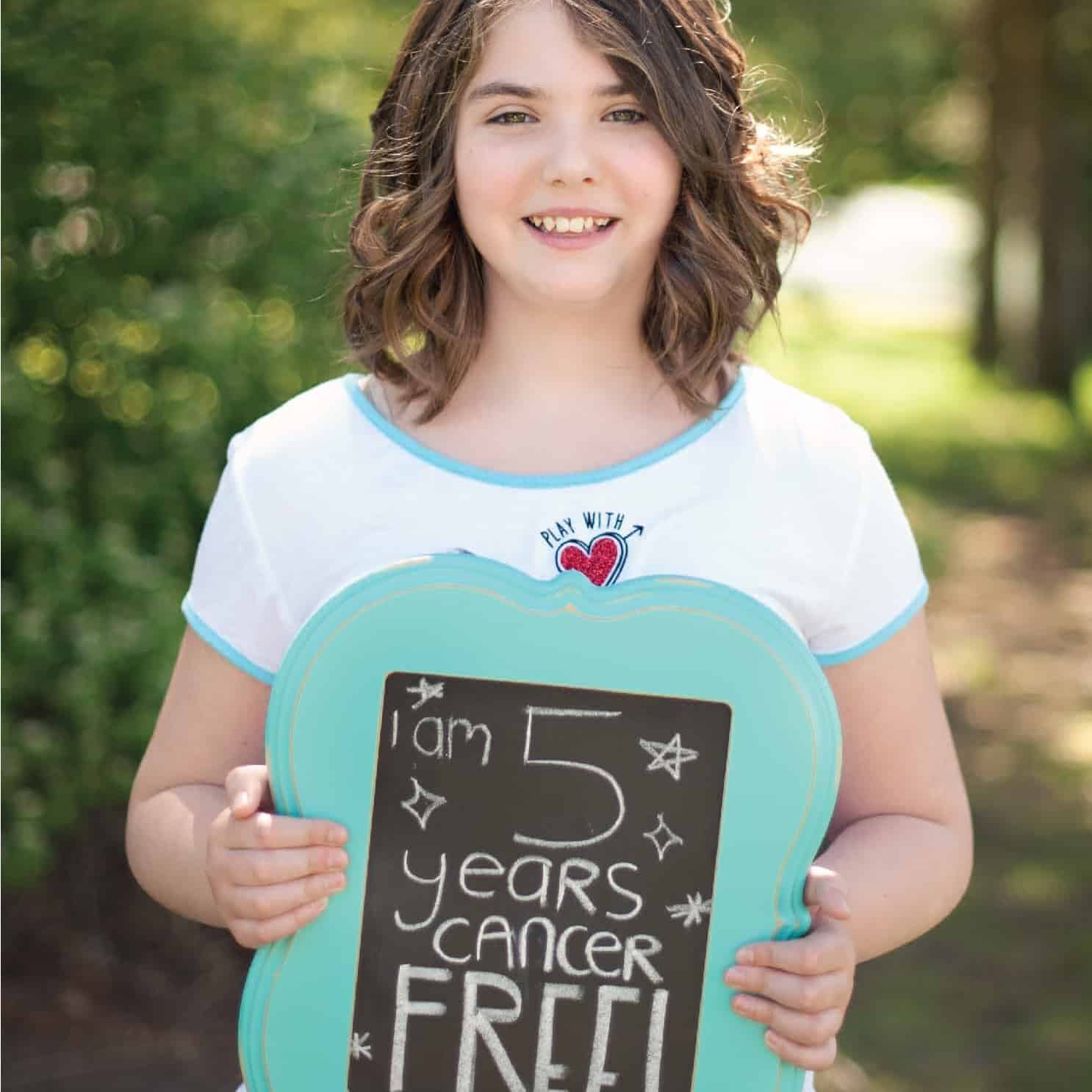
FACT #16
Emily’s immune cells got “sent to boot camp.” Then they helped save her life.
Read more
After being diagnosed with leukemia in 2010, Emily Whitehead tried several rounds of chemotherapy, but none of them worked. Then, in 2012, she enrolled in a clinical trial in which Carl H. June, MD, and his team treated her with a novel immunotherapy strategy that employed CAR (chimeric antigen receptor) T cells.
This CAR T cell immunotherapy involved taking Emily’s own immune cells and equipping them with specialized new receptors (the CARs) specifically designed to target her cancer cells. Then, these powerful new CAR T cells were re-infused back into Emily, and then went to work and eliminated her leukemia. She’s remained cancer-free ever since. (You can read more about Emily’s remarkable story here.)
CAR T cell immunotherapies have been extremely successful against blood cancers such as leukemia and lymphoma, leading to responses in more than half of the patients who have received them. More recently, CAR T cells have been developed that have shown promise in patients with a wider variety of cancers.
The following CRI-funded scientists are figuring out how we might enhance CAR T cell strategies and other T cell-based immunotherapy approaches:

FACT #17
Immunotherapy can help children enjoy longer, healthier lives.
Read more
Children have been receiving immunotherapy in the form of bone marrow transplants for decades. More recently, targeted antibodies and checkpoint immunotherapies have shown success against some forms of childhood cancer. Already, dinutuximab has been approved for children with neuroblastoma, while pembrolizumab has been approved for children with Hodgkin lymphoma.
Scientists are exploring how to improve the effectiveness of bone marrow transplants and molecular-based immunotherapies. Newer immune cell-based approaches, such as the CAR T cells we highlighted previously, have also shown great promise for children, especially those with leukemia.
Furthermore, the side effects of some current therapies can cause long-term, debilitating damage to children who are treated with them. To that end, immunotherapy approaches can potentially offer safer, yet still effective, alternatives for children with cancer. The following CRI-funded scientists are working to improve the immunotherapy options for treating childhood cancers:

FACT #18
Immunotherapy is a pillar of former Vice President Biden’s Cancer Moonshot.
Read more
In 2016, former Vice President Joe Biden launched the National Cancer Moonshot—which featured immunotherapy as a centerpiece—to help accelerate our quest to cure cancer once and for all!
Part of that effort involved the formation of a Blue Ribbon panel—whose membership included James P. Allison, PhD, Elizabeth M. Jaffee, MD, and Laurie H. Glimcher, MD—which laid out ten recommendations for how we can speed up research and development to achieve this goal.
In response to one of the Panel’s recommendations, CRI has spearheaded an effort to create an online database and web resource designed to help basic and clinical researchers navigate immunological data across multiple tumor types. This database will be known as the Cancer Research Institute iAtlas and is the first comprehensive effort in immuno-oncology to address the recent National Cancer Moonshot Blue Ribbon Panel recommendation to establish a pan-cancer immune atlas that would help catalyze new research in cancer immunotherapy.
Image credit: Creative Commons (via Obama White House Archives)

FACT #19
Immunotherapy was the first treatment to extend survival in patients with advanced melanoma.
Read more
Once melanoma has spread to other organs, there isn’t—or rather, wasn’t—much that doctors could do to help patients. Then came James P. Allison, PhD, and his breakthrough work on the immune checkpoint CTLA-4. Dr. Allison’s discoveries led to the development of ipilimumab, an anti-CTLA-4 checkpoint immunotherapy, which was the first treatment of any type that demonstrated the ability to provide a survival benefit for patients with metastatic melanoma in a randomized clinical trial. (You can learn more about how checkpoint immunotherapies work here.)
Since ipilimumab’s FDA approval in 2011, several other immunotherapies have been approved for melanoma patients, including anti-PD-1 checkpoint immunotherapies such as pembrolizumab and nivolumab as well as the oncolytic virus T-Vec. The FDA has also approved the checkpoint immunotherapy combination of ipilimumab and nivolumab for melanoma patients.
After receiving that combination immunotherapy in a clinical trial, Luc V. (pictured) has been able experience and enjoy more time with his family. (You can read Luc’s whole immunotherapy story in our ImmunoCommunity.)
Below are the many CRI-funded scientists who are investigating how to further improve immunotherapy’s benefits for melanoma patients:
Niroshana Anandasabapathy, MD, PhD
Scott J. Antonia, MD, PhD, Georgina V. Long, MD, PhD, Richard A. Scolyer, MD, Mark John Smyth, PhD, FAHMS, and John Stagg, PhD
Nicholas David Huntington, PhD, and Sandra Nicholson, PhD
John M. Kirkwood, MD, and Hassane M. Zarour, MD
Drew M. Pardoll, MD, PhD, Antonia Ribas, MD, PhD, and Cassian Yee, MD

FACT #20
Immunotherapy cured Jimmy Carter’s cancer.
Read more
“[After immunotherapy] … they didn’t find any cancer at all.”
In August 2015, former U.S. president Jimmy Carter announced that his melanoma had spread to his liver and his brain. Several years ago, metastatic melanoma like Carter’s would have been untreatable. Fortunately, Carter’s doctors used a relatively new approach that combined radiation with immunotherapy, a form of cancer treatment that uses the power of the immune system to prevent, control, and eliminate cancer.
First, doctors bombarded Jimmy Carter’s cancer with radiation. The damaged and dying cancer cells likely attracted the attention of immune cells. Then, he received pembrolizumab (KeytrudaⓇ), an anti-PD-1 checkpoint immunotherapy, to support his immune system’s response and help it to completely eliminate the cancer.
In December, three months after Carter first began receiving immunotherapy, the then-91-year-old found out that his tumors were gone. Based on the responses of other cancer patients whose tumors shrank or disappeared after treatment with immunotherapy, there is a high likelihood that President Carter’s immune system will continue to protect him against this type of cancer and that it won’t return.
Success stories like Jimmy Carter’s cancer immunotherapy story reveal that immunotherapy can help older patients.
READ MORE CANCER IMMUNOTHERAPY SUCCESS STORIES
Learn more about checkpoint inhibitors like pembrolizumab that Jimmy Carter received for his cancer treatment, immunotherapy treatments for different types of cancer, and immunotherapy clinical trials.
Supporting the future of cancer immunotherapy
Your support of the Cancer Research Institute directly impacts the discovery and development of powerful immunotherapies for all types of cancers, funding more breakthroughs and saving more lives. Donate today to CRI and explore other ways to join our cause.
With your support, the Cancer Research Institute can, in turn, fund doctors and scientists leading immunology research and working to develop immunotherapy as a cure for all cancers. The following CRI-funded scientists are evaluating the clinical benefits of other combination approaches that utilize immunotherapy:
- James P. Allison, PhD, and others are leading the SU2C-CRI Cancer Immunology Translational Research Dream Team, which is performing comprehensive experiments aimed at improving immunotherapy’s ability to improve patient outcomes.
- Scott J. Antonia, MD, PhD, and others are leading efforts to address the impact of adenosine in the tumor environment.
- Nina Bhardwaj, MD, PhD, and Sacha Gnjatic, PhD, are investigating the relationship between checkpoint immunotherapy, chemotherapy, and mutations in advanced bladder cancer.
- Joshua Brody, MD, is exploring if combination immunotherapy in lymphoma patients can enhance dendritic cell activity.
- John M. Kirkwood, MD, and Hassane M. Zarour, MD, are evaluating an approach using the TIGIT receptor that may be able to complement PD-1 immunotherapy.
Discover different aspects of the science, history, development, and impact of cancer immunotherapy with 30 facts about immunotherapy, presented as part of our fifth annual Cancer Immunotherapy Month.
Stay up-to-date with the latest in cancer immunotherapy news by signing up for our e-newsletter.
FACT #21
Weakened tumor cells can provide valuable intel to immune cells.
Read more
Yesterday, we discussed how the combination of immunotherapy and radiation helped former U.S. president Jimmy Carter overcome melanoma. The logic behind that strategy was that the radiation would damage cancer cells and cause them to release their inner contents and stimulate an immune response against the remaining cancer cells.
Another immunotherapy strategy—a vaccine called GVAX—takes this concept a step further. It also involves exposing tumor cells to radiation, but with GVAX, tumor cells are removed from patients and then irradiated “in a dish.” These cells are also modified so that they produce a molecule called GM-CSF, which is intended to support a robust, tumor-specific immune response once these cells are transferred to patients.
GVAX is currently being evaluated for several cancers in clinical trials, including some in combination with checkpoint immunotherapies.
FACT #22
Viruses and bacteria can be reprogrammed to help eliminate cancer.
Read more
In addition to modified tumor cells, bacteria and viruses can also be equipped with pro-immune / anti-cancer abilities. Several different virus-based strategies exist, the most successful being T-VEC, an oncolytic virus immunotherapy that’s already FDA-approved for melanoma (more on this tomorrow).
Another approach–CRS-207—uses de-fanged but living bacteria (L. monocytogenes) to vaccinate patients.
CRS-207 cells are changed in two important ways. First, they have been “double-deleted,” which renders them less dangerous to normal cells. Second, they have been loaded with the recipe for a specific protein—mesothelin—that’s expressed by several types of cancers. This vaccination is designed to provide the immune system with the information necessary to mount a tumor-specific response.
The therapeutic benefits of CRS-207 in combination with other immunotherapies, including GVAX and checkpoint inhibitors, are currently being evaluated in clinical trials for patients with gastro-esophageal cancer, ovarian cancer, pancreatic cancer, and mesothelioma.

FACT #23
“My immune system was awakened and it knew exactly where to go.”
Read more
After prior therapies proved ineffective for her malignant melanoma, Janie Ferling enrolled in a clinical trial led by Antoni Ribas, MD, PhD, and received a combination of two distinct immunotherapies that had already been approved individually.
In addition to an anti-PD-1 checkpoint inhibitor, Janie received T-VEC, a re-engineered herpes virus designed to do three things: infect tumor cells, make them burst, and attract the attention of the immune system. T-VEC-infected cells, like GVAX tumor cells, possess the “recipe” for GM-CSF, a protein that can boost immune cell production and activity. (David M. Reese, MD, was instrumental in the development of T-VEC.)
After many months of treatment, Janie’s tumors shrank significantly and, although they aren’t all completely gone, appear to be stable. Even so, it hasn’t stopped her from “traveling the world and making more memories with her son.”
FACT #24
Some bacteria inside of us appear to make immunotherapy more effective.
Read more
Trillions of bacteria reside in a person’s intestinal tract, and they perform several important duties, most notably helping with digestion. More recently, the influence of the microbiome—all of a person’s bacteria—on both the immune system and cancer has come to be better understood and appreciated, and represents a promising avenue for improving our ability to treat as well as prevent cancer.
In our intestines, the balance between “good” and “bad” bacteria, and their interactions with the immune system, are especially important for tissue health. Certain bacteria and the toxins they produce are associated with biofilm formation, inflammation, and the development of colorectal cancer (CRC). Research being conducted in this area could help improve our ability to prevent CRC.
In addition to the role bacteria play in the formation of cancer in our intestines, they also appear to influence our immune system’s ability to eliminate tumors, no matter where they are in the body. Recent work involving James P. Allison, PhD, and Padmanee Sharma, MD, PhD, showed that in checkpoint immunotherapy-treated patients with metastatic melanoma, different bacterial species were found in the patients who responded compared to those who didn’t respond to the treatment.
Last but not least, bacteria can also be used as treatments themselves that stimulate the immune system against cancer. In addition to BCG (a tuberculosis vaccine that is approved for bladder cancer) and CRS-207 (a modified bacteria being evaluated for several types of cancer) let’s not forget that Dr. William B. Coley’s first immunotherapy also involved bacteria.
The following CRI-funded scientists are working to understand the relationship between bacteria, the immune system, and a variety of cancers, to aid the development of improved strategies for patients:

FACT #25
Immunotherapy works for dogs too!
Read more
As many of you know, cancer affects dogs too, and is, in fact, the most common cause of death in older dogs (ages 10+). Fortunately, immunotherapy’s benefits are beginning to be extended to dogs as well, and the University of Pennsylvania is leading these efforts through their Penn Vet Cancer Center, which is directed by Ellen Pure, PhD, and their Mason Canine Cancer Immunotherapy Research, which is led by Nicola J. Mason, PhD, B.Vet.Med.
Dr. Mason is pictured above with Lewis, a golden retriever with osteosarcoma (bone cancer) who was treated in one of Dr. Mason’s immunotherapy clinical trials. After undergoing a limb spare procedure (that allowed him to avoid total amputation), Lewis received a genetically modified bacteria similar to CRS-207 that targets a tumor-specific protein. This bacterial vaccine is designed to stimulate his immune system against any hidden metastases as well as endow his immune system with “memory” so that it can provide protection against the cancer should it ever return.
Vaccines aren’t the only immunotherapies Dr. Mason’s team uses to treat dogs. Two examples are Kobe (below, left), who is being treated with an approach that enables his immune system to produce a tumor-targeting antibody, while Harley has been treated with anti-CD20 CAR (chimeric antigen receptor) T cell immunotherapy for his leukemia.
FACT #26
Only immunotherapy is approved to treat tumors regardless of their origin.
Read more
On May 23, 2017, a checkpoint immunotherapy, the anti-PD-1 pembrolizumab, became the first cancer treatment (of any type) to be approved by the FDA without any organ-specific strings attached.
Instead, this immunotherapy’s approval depends on the stability of a tumor’s genome.
Now, any adult or child with metastatic cancer that has become genetically unstable (categorized by the MSI-hi or dMMR biomarker) can receive this immunotherapy, regardless of their tumor’s physical origin, if other available treatments aren’t working.
In the clinical trial that led to this approval, patients with fifteen different tumor types were represented and overall nearly 40% of patients responded. These tumors likely respond well to immunotherapy because their high levels of DNA mutations produce abnormal proteins (neo-antigens) that the immune system can target due to their “foreign” appearance.
Oftentimes, immune cells have already even mounted a response against a tumor’s neo-antigens, but are exhausted via the PD-1/PD-L1 pathway. In that case, a checkpoint immunotherapy that blocks the PD-1/PD-L1 pathways can tilt the scales in the immune system’s (and patient’s) favor.
FACT #27
Immunotherapy can empower your immune system against cancer
Read more
The immune system is naturally equipped to protect us against cancer. Cytotoxic T lymphocytes—otherwise known as killer T cells—are especially effective at targeting tumors. However, cancers sometimes figure out how to outsmart the immune system and protect themselves.
Immunotherapy aims to reverse that situation.
Over the next thirty days, we’ll be highlighting a wide scope of immune-based approaches that are already improving outcomes for patients. Many of these treatments work by either directly or indirectly enhancing the activity of T cells (the blue/white cells pictured aboved).
Much of what we know about the immune system and its relationship to cancer was discovered by scientists affiliated with the Cancer Research Institute (CRI), which since 1953 has served as the world’s leading (and for several decades only) nonprofit organization dedicated exclusively to transforming cancer patient care by advancing immunotherapy and the science behind it.
FACT #28
Focused ultrasound is being used to disrupt tumor defenses.
Read more
Ultrasound technology has many applications, most commonly during pregnancy. Other strategies can amplify its power for use against other diseases, including cancer. While high-intensity ultrasound waves can be used to destroy tumors directly, its greatest benefit against cancer may prove to be its ability to support the immune system’s activity.
Checkpoint pathways aren’t the only way a tumor can be protected from immune responses. Other factors can physically prevent immune cells and immunotherapy drugs from even getting to the tumor. The blood-brain barrier can shield tumors in the brain—both those that started there as well as those that have spread there from another tissue. It can also be hard to deliver immunotherapy agents to tumors, no matter their location, because of “leaky” blood vessels that cause a pressure imbalance.
To address this, scientists are utilizing an image-guided approach that uses focused ultrasound, to manipulate microbubbles and disrupt these defenses. A project being supported through the partnership between CRI and the Focused Ultrasound Foundation is now evaluating this strategy for its ability to enhance immune responses against tumors both alone and in combination with other immunotherapies.

FACT #29
The immune system uses cytokines to “sound the alarm.”
Read more
Like a well-trained fire station crew, the immune system can quickly spring into action at the “sound” of danger. Instead of alarms though, immune cell substances called cytokines (taken from the Greek words for cell and movement) trigger immune cell movement and get them where they need to be to respond effectively. Cytokines come various forms that can allow for responses against a variety of threats, including cancer.
In addition to affecting immune cells, cytokines can also act directly on other types of cells, both healthy and diseased. One cytokine, tumor necrosis factor alpha (TNF-α), was named (by Dr. Lloyd J. Old) for its ability to induce cancer cell death. TNF-α also plays important roles in responses to bacteria and viruses, helping to eliminate infected cells. Another cytokine, interferon gamma (IFN-γ), is an important stimulator of adaptive immune responses that are capable of targeting tumors with incredible precision. The activity of IFN-γ was crucial in the work of Robert D. Schreiber, PhD, that validated the immunosurveillance hypothesis.
Other CRI scientists who have made important contributions to our understanding of cytokines and immune cell migration include:

FACT #30
CRI has transformed immunotherapy into a global phenomenon.
Read more
In 1953, thanks to a $2,000 grant from Nelson Rockefeller, the Cancer Research Institute (CRI) was founded by Dr. William B. Coley’s daughter, Helen, with the goal of better understanding how a bacterial treatment her father created—the first-ever cancer immunotherapy—could be used to save the lives of more people. Theorizing that the treatment likely worked in some patients by stimulating an immune response against cancer, CRI turned its focus to basic immunology to build a foundation of knowledge that would one day make possible effective immunotherapies for more patients.
The return on that investment in CRI has been immense. Since then, CRI has funded more than $344 million in immunotherapy research and supported more than 3,000 scientists, who have made crucial discoveries and helped advance immunotherapy efforts in countries across the world (pictured). More importantly, the immunotherapies that CRI scientists helped develop have saved the lives of many patients—both young and old—with previously untreatable cancer, enabling them to enjoy more years of their lives.
While the range of patients benefiting from immunotherapy continues to grow, it doesn’t work for everyone yet. But some of the best scientific minds in the world are committed to changing that. Globally, there are thousands of immunotherapy clinical trials ongoing for nearly all types of patients and all types of cancer, and the progress that’s being made (which we’ve highlighted in our 30 Immunotherapy Facts of the Day) should give us all reason to believe in the possibility of a Future Immune to Cancer.
Join CRI in Shaping the Future of Immunotherapy
Support the pioneering work of CRI in advancing immunotherapy.
