The American Cancer Society’s Cancer Statistics 2026 report tells a remarkable story of scientific breakthroughs – particularly in immunotherapy and targeted treatments – that are extending lives once thought impossible to save.
In the United States, the five-year relative survival rate for all cancers has reached a historic milestone of 70% during 2015–2021 – up from 49% in the mid-1970s. Perhaps most striking are the dramatic gains in survival for advanced, metastatic cancers that were often considered untreatable just a generation ago.
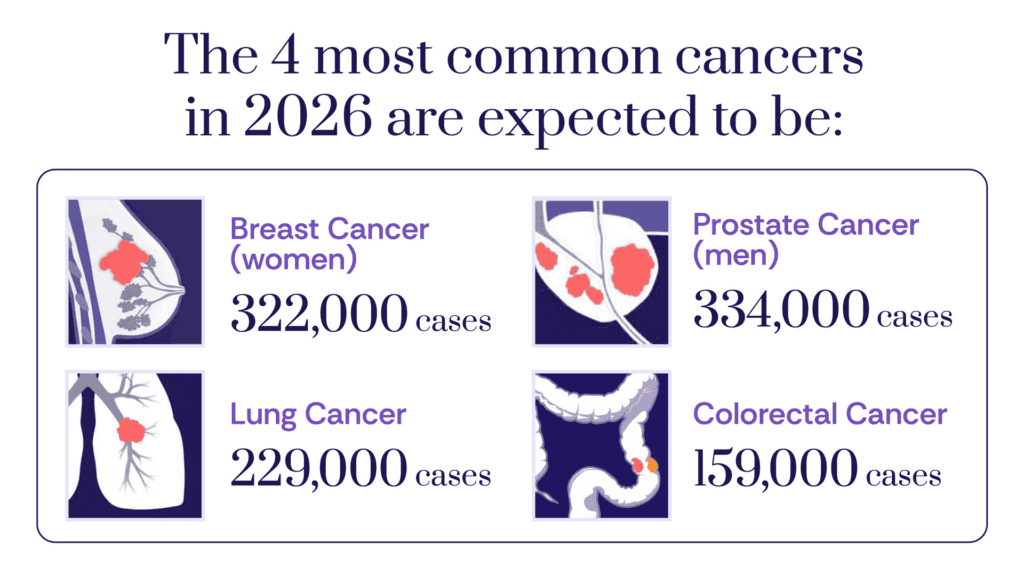
Cancer Burden: By the Numbers
Cancer is the second leading cause of death in the U.S. overall, but is the leading cause of death in men aged 60–79 and women aged 40–79. In 2026, an estimated 2.1M Americans will be diagnosed with cancer, with an estimated 626,000 deaths — representing about 5,800 new cases and 1,720 deaths every single day. Lung cancer alone is estimated to cause more deaths than the second- and third-deadliest cancers – colorectal and pancreatic – combined.
Immunotherapy Is Helping Rewrite Survival Curves
The report documents extraordinary five-year survival gains for several advanced cancers, driven by breakthrough treatments including immunotherapy and targeted therapies.
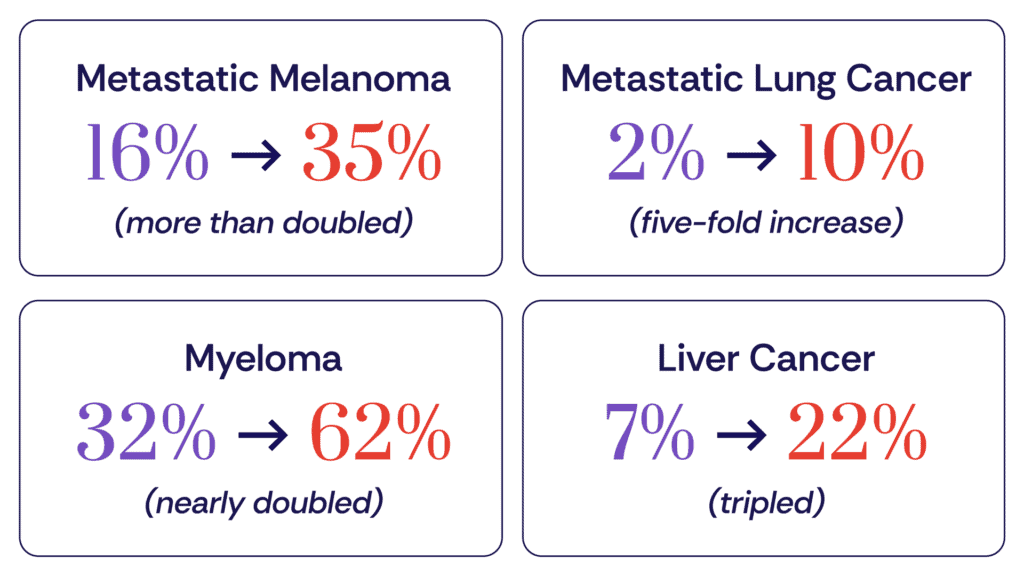
These aren’t marginal improvements. They represent fundamental shifts in what’s possible for patients with advanced disease–shifts enabled by decades of research into how to harness the immune system and target cancer’s vulnerabilities.
“When we look at metastatic melanoma survival improving from 16% to 35% in just 25 years, we’re witnessing the direct impact of immune checkpoint inhibitors like anti-PD-1 and anti-CTLA-4 therapies. These are the breakthroughs that the Cancer Research Institute has championed for decades — supporting the fundamental science that made immunotherapies a reality.”
– Antoni Ribas, MD, PhD, Professor at UCLA and CRI Scientific Advisory Council member
Progress Is Real, But Uneven
From 1991 to 2023, the cancer death rate declined by 34%, translating to an estimated 4.8 million deaths averted. This progress stems from a variety of factors, including reduced smoking, earlier detection, and improved treatments—including the immunotherapy revolution.
Despite decades of advancement, the report also highlights areas where progress has been slower and uneven—underscoring the need for continued research and innovation.
Young adults face rising cancer rates:
- Colorectal cancer incidence is increasing 2.9% per year in people under 50, even as overall rates declined 0.9% per year over the past decade due to screening and early detection in older adults
- Breast cancer incidence is rising overall (1% per year since 2013), with a steeper increase in women under 50 (1.4% per year)
Geographic gaps are widening:
- Cancer death rates range from 122 per 100,000 (UT, HI) to 180 (KY) – a 48% gap driven largely by lung cancer and mirrors smoking prevalence
- Cervical cancer incidence varies two-fold across states (6 per 100,000 in MA/NH vs. 14-15 in MS/OK/AR/LA), despite being largely preventable
- HPV vaccination coverage ranges from 38% in MS to 84% in RI, directly mirroring cervical cancer rates
Racial and ethnic inequities persist – driven by systemic barriers:
The report emphasizes certain disparities that stem from a “higher prevalence of risk factors, medical mistrust, and lack of insurance, which hinders access to high-quality health care.”
Even after controlling for stage at diagnosis and socioeconomic status, survival gaps remain – with the “largest contributor” being “less access to high-quality care across the cancer continuum, from prevention to diagnosis and treatment.”
As a result, the report notes the following:
- Black men have prostate cancer death rates 2–4 times higher than other groups
- American Indians/Alaska Natives face double the mortality rates for kidney, liver, stomach, and cervical cancers
- Black women have uterine death rates twice as high as White women
- Asian, Black, and Hispanic patients are less likely to receive recommended genetic testing–a gateway to targeted therapies
What the Report Means for Immunotherapy
The survival gains documented in this report validate decades of investment in cancer immunology. But they also underscore how much work remains:
- Expand immunotherapy to more cancer types: While melanoma and lung cancer have seen dramatic gains, many cancers remain resistant to current immunotherapies. The five-year survival rate for pancreatic cancer, for example, remains at just 13%, and uterine corpus cancer mortality has been rising for 26 consecutive years.
- Close the early detection gap: Cancer stage at diagnosis remains critical – only 17% of pancreatic cancers and 28% of lung cancers are caught at the localized stage. Despite lung cancer screening reducing mortality by up to 24% in high-risk individuals, only about 18% of eligible patients received it, with even lower rates among those under 60 (<13%) and Native Americans (14%). Earlier detection gives immunotherapy its best chance to work, as localized lung cancer has 65% five-year survival compared to 10% for distant-stage disease.
- Ensure broad access to innovation: Scientific breakthroughs only save lives if patients can benefit from them. The report shows wide variation in cancer incidence and outcomes across the U.S., reflecting geographical differences in prevention, early detection, and availability of high-quality care. Ensuring that advances in immunotherapy and precision medicine reach patients everywhere remains a critical challenge.
- Accelerate research for pediatric cancers: While five-year survival rates for some childhood cancers have improved dramatically (for example, leukemia has improved from 50% in the mid 1970s to 89% in 2015–2021), one in six survivors faces major cardiovascular events by age 50, highlighting the need for gentler, more effective treatments.
- Protect research funding: The report warns that continued progress is threatened by proposed federal cuts to cancer research and health insurance. Advances in immunotherapy are the result of decades of investment in foundational scientific research–and future breakthroughs will be impossible without the same long-term commitment. The stakes couldn’t be higher: cutting research funding now would halt progress precisely when patients need it most.
The Road Ahead
The Cancer Statistics 2026 report is a reminder that research works. The immunotherapy revolution didn’t happen overnight–it was built on decades of basic science funded by institutions like the National Cancer Institute and philanthropic organizations such as the Cancer Research Institute (CRI).

But the same data that celebrates progress also reveals how far we still have to go. Pancreatic cancer survival remains stagnant at 13%, meaning an estimated 185 Americans diagnosed today face essentially unchanged odds. Uterine corpus cancer mortality continues its 26-year climb.
Every patient deserves access to the latest advances in immunotherapy. Every cancer type deserves the research investment needed for breakthroughs. And every community – regardless of race, ethnicity, or ZIP code – deserves equal access to life-saving cancer care.
The immune system holds extraordinary power to fight cancer. Continued research is essential to unlocking that power and extending its promise to patients everywhere.
