Immune to Cancer: The CRI Blog
-
Tecelra® Receives FDA Approval: A New Milestone in Cancer Treatment
On August 2, 2024, the U.S Food and Drug Administration (FDA) approved Tecelra® (afamitresgene autoleucel), a gene… -
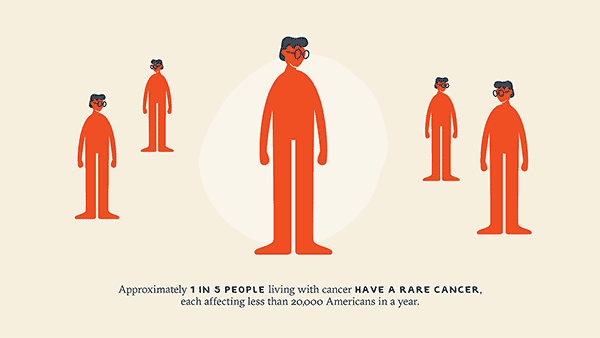
Immunotherapy for Rare Cancers: New Horizons and New Hope
Rare cancers are more common than many realize. Collectively, they account for nearly one in four cases… -
Sarcoma Awareness Month: 2021 Immunotherapy Research Updates
This July for Sarcoma Awareness Month, we look at new research, new treatments, and how we’re working…
-

Sarcoma Awareness Month: Tackling Chordoma with Dr. Cassian Yee
CRI-Chordoma Foundation CLIP Investigator Dr. Cassian Yee is exploring how to design cell therapies for patients with…
-
Sarcoma Awareness Month: 2020 Immunotherapy Research Updates
This July for Sarcoma Awareness Month, we look at genetics and genomics, new research, and how we’re…
-
Sarcoma Awareness Month: Immunotherapy for Sarcoma 2019 Research Update
July is Sarcoma Awareness Month. As a rare and difficult-to-treat form of cancer, effective drug treatments are…
-
Behind the Changing Landscape of Chordoma Research and Treatment with Joan Levy, PhD
Dr. Joan Levy, director of research at the Chordoma Foundation, discusses the current state of chordoma research…

