Immune to Cancer: The CRI Blog
-
30 Days of CRI Impact
Throughout Cancer Immunotherapy Month in June we’ll be highlighting the 30 most important CRI-funded breakthroughs.
-
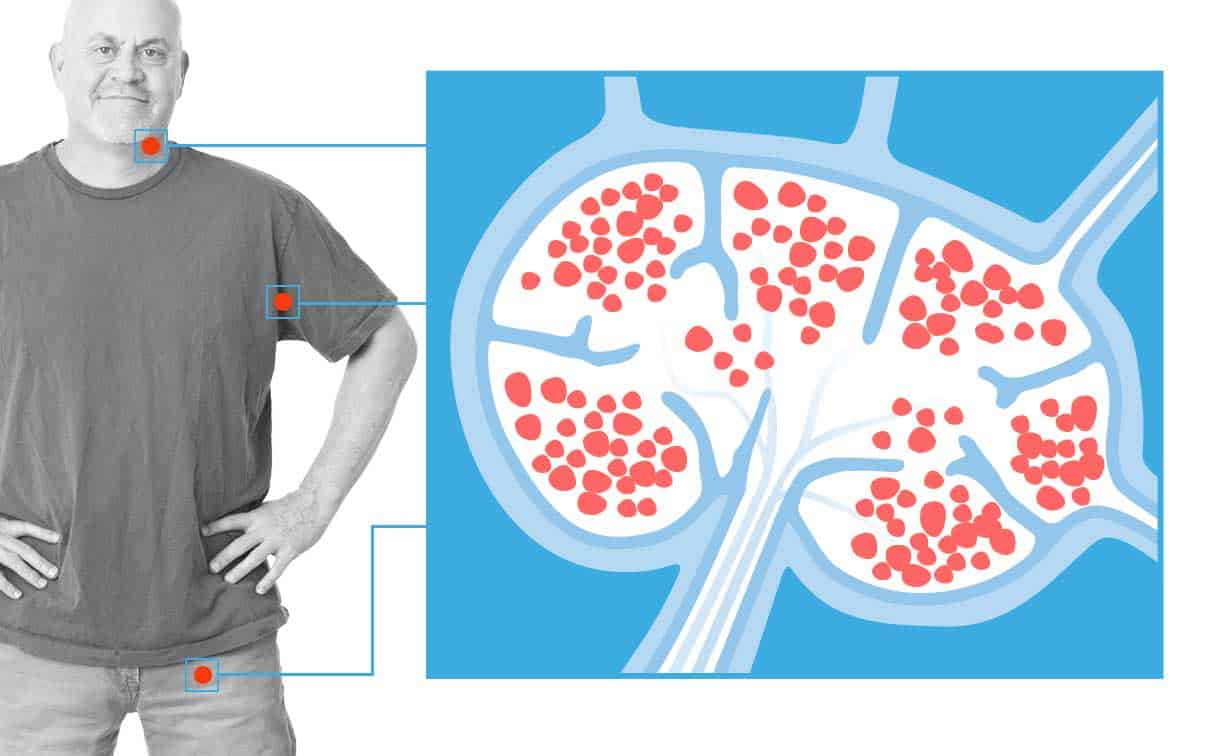
Second CAR T Cell Immunotherapy Approved for Patients with Lymphoma
A second CAR T cell immunotherapy targeting the CD19 receptor was approved by the FDA for patients…
-

AACR18 Recap: Immunotherapy in the Spotlight
Immunotherapy took center stage and dominated the headlines at AACR18.
-
AACR18 Day 3 Update: Lung Cancer in the Spotlight, Immune Memory, and New Immunotherapy Biomarkers
Day 3 of AACR18 highlighted potentially “practice-changing” immunotherapy breakthroughs in lung cancer, and insights into the factors…
-

AACR18: Driving Innovative Cancer Science to Patient Care
This year’s annual AACR meeting boasts an impressive lineup of presentations highlighting both clinical and preclinical immunotherapy…
-

How Man’s Best Friend Could Help Us Cure Cancer
Cancer very is similar in dogs and humans, and scientists are now exploring how cures for one…
-
Immunotherapy is Here to Stay: Looking Back at this Year’s Breakthroughs
Important immunotherapy breakthroughs benefited patients immensely in 2017 and paved the way for even more advances in…
-
Immunotherapy Shines at ASH and ESMO IO Conferences
Two conferences, one in America and one in Europe, highlight immunotherapy's continued progress and the important contributions…
-
CAR T Cell Immunotherapy Approved for Adult Non-Hodgkin Lymphoma Patients
FDA approves new immunotherapy–Yescarta (axicabtagene ciloleucel)–for adult patients with relapsed or refractory non-Hodgkin large B cell lymphoma.