Immune to Cancer: The CRI Blog
-

Immunotherapy Approvals in 2015
In 2015, the FDA approved more immunotherapies than in any prior year.
-

Top 5 Cancer Immunotherapy Stories of 2015
Here’s what you, our online community, thought were the biggest stories of 2015.
-
CRI Scientific Advisory Council Member Elected NAI Fellow
Pramod K. Srivastava, MD, PhD, a CRI Scientific Advisory Council member, was elected a Fellow in the National…
-
CRI Partners with the Lymphoma Research Foundation to Improve Immunotherapy Evaluation Guidelines for Lymphoma
CRI, LRF work towards better immunotherapy guidelines for lymphoma.
-
Second Antibody FDA-Approved Against Multiple Myeloma
Elotuzumab (Empliciti) is the second antibody for myeloma approved in just two weeks.
-

Fourth Annual #GivingTuesday a Record Success
Here’s to you—for kicking off this season with a gift for a good reason. The Cancer Research Institute…
-
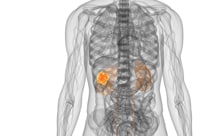
Nivolumab Becomes First FDA-Approved Checkpoint Blockade for Kidney Cancer
New hope now exists for patients with advanced kidney cancer.
-
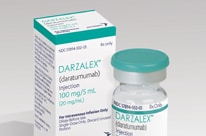
First Monoclonal Antibody for Multiple Myeloma Receives FDA Approval
Daratumumab (Darzalex®) is the first approved immunotherapy that targets CD38—a protein found in most multiple myeloma cells.
-

CRI Feels the LOVE and Wins $250,000!
Thanks to our donors, we won second place in the Revlon LOVE IS ON Million Dollar Challenge.

Join CRI in Shaping the Future of Immunotherapy
Support the pioneering work of CRI in advancing immunotherapy.