Immune to Cancer: The CRI Blog
-

Immunotherapy Makes History with Latest Cancer Approval
The FDA’s biomarker-based approval of Keytruda was the first of its kind
-
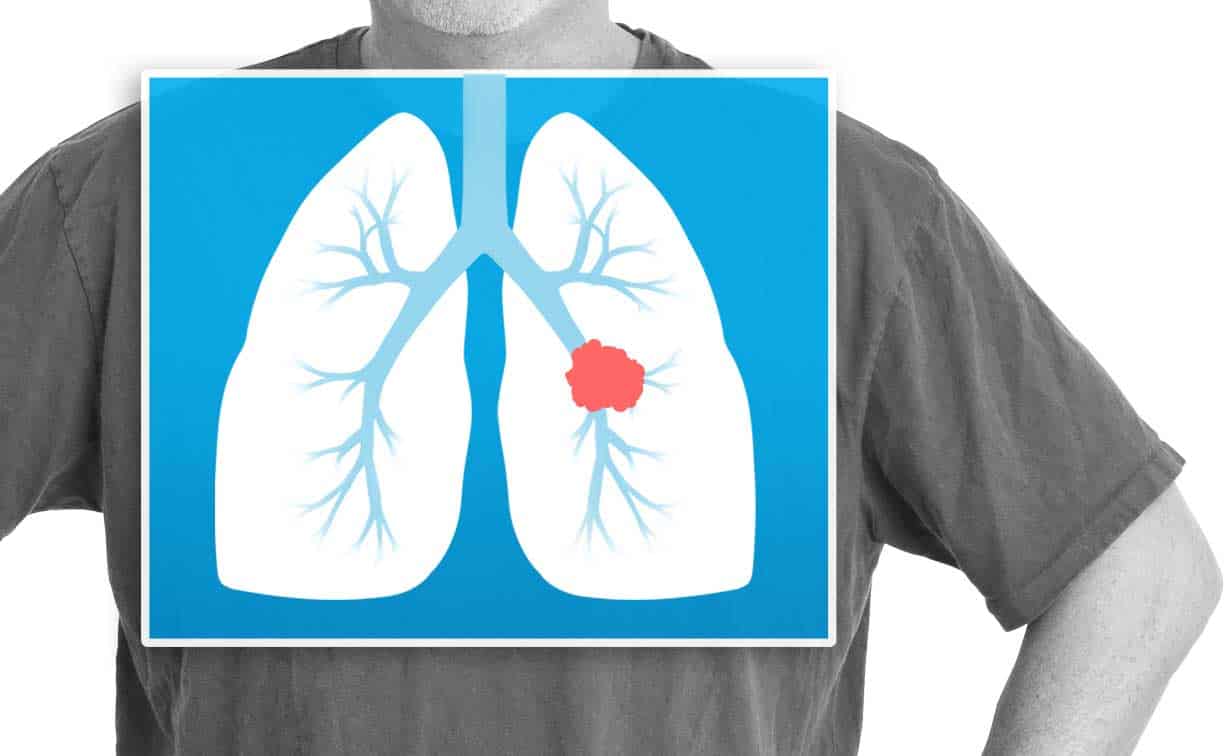
FDA approves Keytruda combo for first-line lung cancer treatment
Patients in the U.S. who have advanced nonsquamous non-small cell lung cancer can now receive Keytruda as…
-
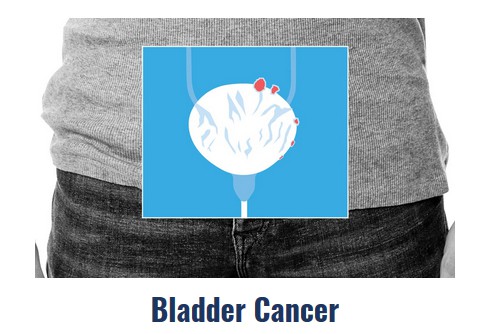
Durvalumab Becomes Newest Immunotherapy Approved for Advanced Bladder Cancer
Durvalumab marks AstraZeneca’s first immunotherapy approval
-
Meet Ariella: Captain of Team Natural Killer Cells in the Answer to Cancer 5K Survivors Walk
Ariella was diagnosed with Hodgkin lymphoma in 2010, but treatment with the immunotherapy drug nivolumab changed everything and…
-

NY-ESO-1 Identified as a Promising Vaccine Target in Ovarian Cancer Patients
New study reveals effectiveness of immunotherapy vaccine against aggressive ovarian tumors.
-
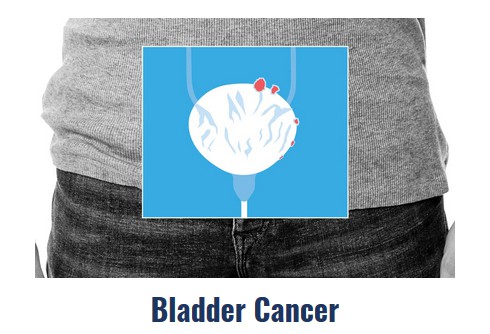
Atezolizumab approved as first-line therapy for chemotherapy-ineligible bladder cancer patients
Atezolizumab’s third approval in the last year provides an important treatment option for a neglected population of…
-
TIME Magazine Names CRI’s Dr. James Allison One of 100 Most Influential People
TIME magazine honors Dr. Allison’s development of the first checkpoint immunotherapy, which has led to a revolution…
-

Meet Kathy: 100-Mile Rider in 2017 Answer to Cancer
On June 10, 2017, Kathy will ride for her husband, T.J.
-

PIMCO: Supporting a Future Immune to Cancer
PIMCO sponsors the 5K Family Fun Run/Walk of the 2nd annual Answer to Cancer and fields an…

Join CRI in Shaping the Future of Immunotherapy
Support the pioneering work of CRI in advancing immunotherapy.