Immune to Cancer: The CRI Blog
-

Corporate Social Responsibility Gives Cancer Immunotherapy Research a “Wonderful Edge”
Cupid Intimates spearheaded a corporate social responsibility initiative that generated a $10,000 donation to CRI’s immunotherapy research…
-
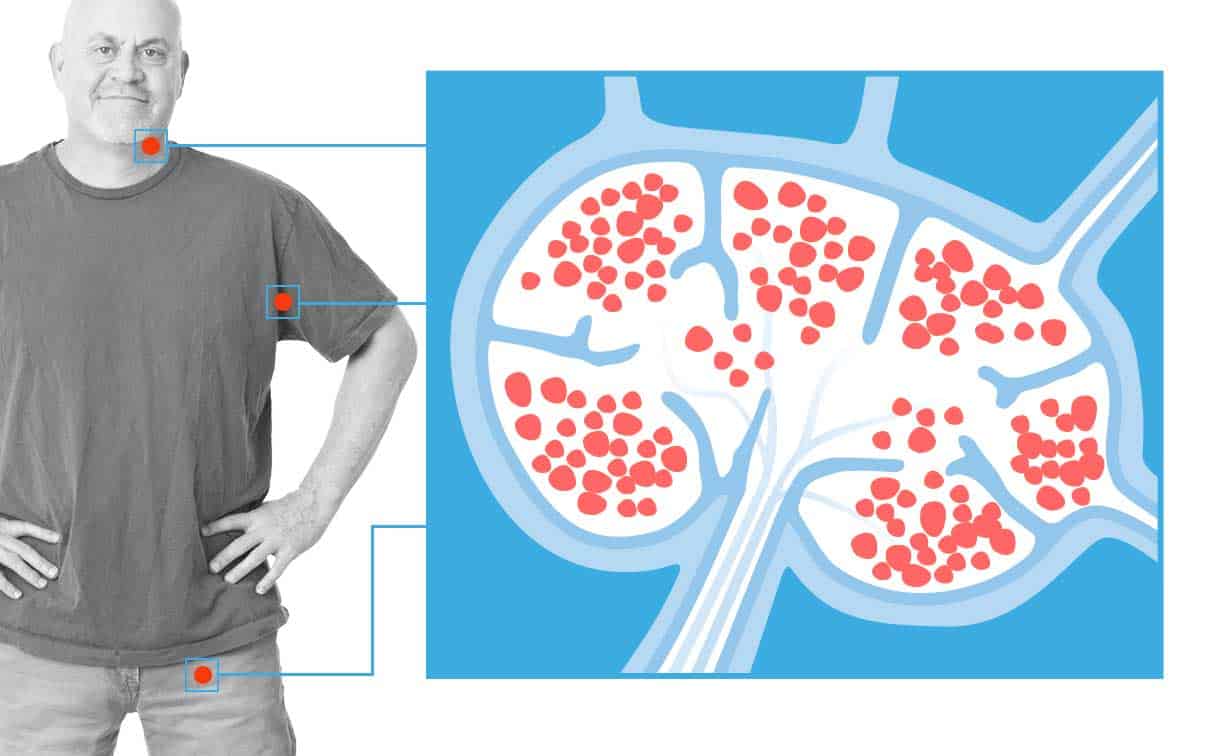
CAR T Cell Immunotherapy Approved for Adult Non-Hodgkin Lymphoma Patients
FDA approves new immunotherapy–Yescarta (axicabtagene ciloleucel)–for adult patients with relapsed or refractory non-Hodgkin large B cell lymphoma.
-

CRI featured in USA Today’s End-of-Year Charity Giving Advice
CRI among 12 top-rated cancer charities within a subset of organizations doing good work in breast cancer…
-

Checkpoint Immunotherapy Approved for Patients with Stomach, Gastroesophageal, and Liver Cancer
Previously treated patients with these cancer types can now receive anti-PD-1 immunotherapy; nivolumab for HCC and pembrolizumab…
-
CICON17 Day 4 Recap: The Tumor Microenvironment and Oncolytic Viruses
The final day of CICON17 explored two newer immunotherapy approaches that reflect broader understanding of tumor-immune interactions…
-
CICON17 Day 3 Recap: Combinations, Checkpoint Immunotherapy Resistance, Immunomodulators, and Microbiota
Day 3 of the CICON17 focused on combination therapies, immunomodulators, overcoming immune suppression, and the role bacteria in…
-
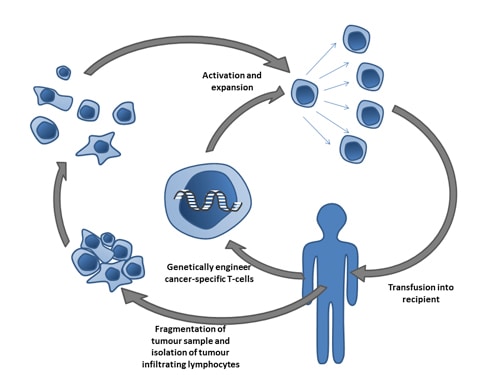
CICON17 Day 2 Recap: Biomarkers, Novel Agents, and Adoptive Cell Therapies
Day 2 of CICON17 offered a focused exploration of the latest discoveries and data from scientific studies…
-
CICON17 Day 1 Recap: Neoantigens, Vaccines, and Overcoming Immunotherapy Resistance
Day 1 of CICON17 explored how to identify and target neoantigens with vaccines and other strategies to…
-
FDA Approves First-In-Class CAR T Cell Immunotherapy for Leukemia
Children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL) can now receive the CD19-targeting CAR T…

Join CRI in Shaping the Future of Immunotherapy
Support the pioneering work of CRI in advancing immunotherapy.