Immune to Cancer: The CRI Blog
-

The Power of Transformational Gifts
Atlantic Philanthropies, a large charitable foundation, recently released a report summarizing the group's investments in cancer and…
-
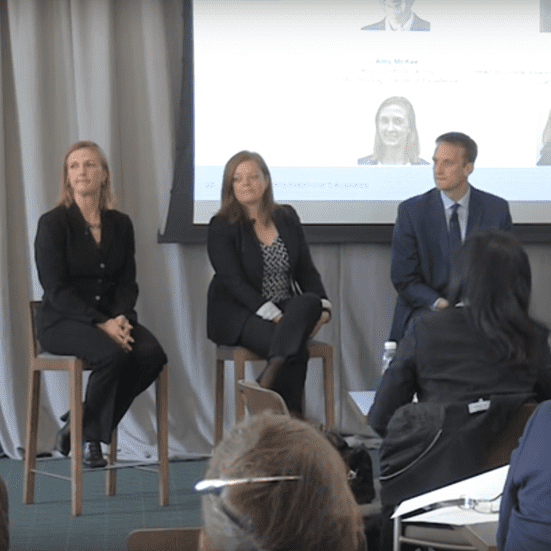
Advocating for Innovative Clinical Trial Design
Laura Pearce, CRI consultant and head of clinical alliances, participates in a clinical trials panel on platform…
-

AACR18 Recap: Immunotherapy in the Spotlight
Immunotherapy took center stage and dominated the headlines at AACR18.
-

AACR18 Update: Lloyd J. Old Award, Overcoming Immunotherapy Resistance, and the Importance of Dendritic Cells
Day 4 at AACR18 was a special day for CRI’s Dr. Antoni Ribas, and highlighted his and…
-
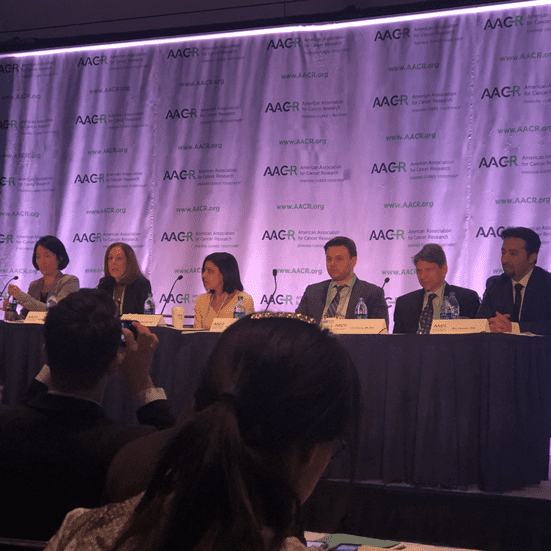
AACR18 Day 3 Update: Lung Cancer in the Spotlight, Immune Memory, and New Immunotherapy Biomarkers
Day 3 of AACR18 highlighted potentially “practice-changing” immunotherapy breakthroughs in lung cancer, and insights into the factors…
-

Checkpoint Immunotherapy Combination Approved for First-line Treatment of Advanced Kidney Cancer
The combination of checkpoint immunotherapies against PD-1 and CTLA-4 significantly improved survival and response rates compared to…
-

AACR18 Day 2 Update: What’s Next for Cancer Immunotherapy?
CRI scientists kicked off the opening plenary session at AACR18 and discussed several promising avenues of immunotherapy…
-

AACR18: Driving Innovative Cancer Science to Patient Care
This year’s annual AACR meeting boasts an impressive lineup of presentations highlighting both clinical and preclinical immunotherapy…
-

Healthcare Experts Tackle Tough Challenges at Fortune Brainstorm HEALTH 2018
CRI CEO highlights next steps in immunotherapy’s advancement at Fortune’s premier healthcare conference

Join CRI in Shaping the Future of Immunotherapy
Support the pioneering work of CRI in advancing immunotherapy.