Throughout the month of June and in celebration of the Cancer Research Institute's 65 years of pioneering leadership in the field of cancer immunology, we are sharing 30 of the most important scientific breakthroughs made possible with CRI funding. Just like last week, we’ll provide some background and context for these advancements.
This week we're highlighting the development of checkpoint immunotherapy and other findings that have helped improve its use in the clinic. For more on cancer immunotherapy and our 30 Days of CRI Impact, be sure to follow #CIM18 on Twitter.
1995: Drs. Frank Borriello and Arlene Sharpe
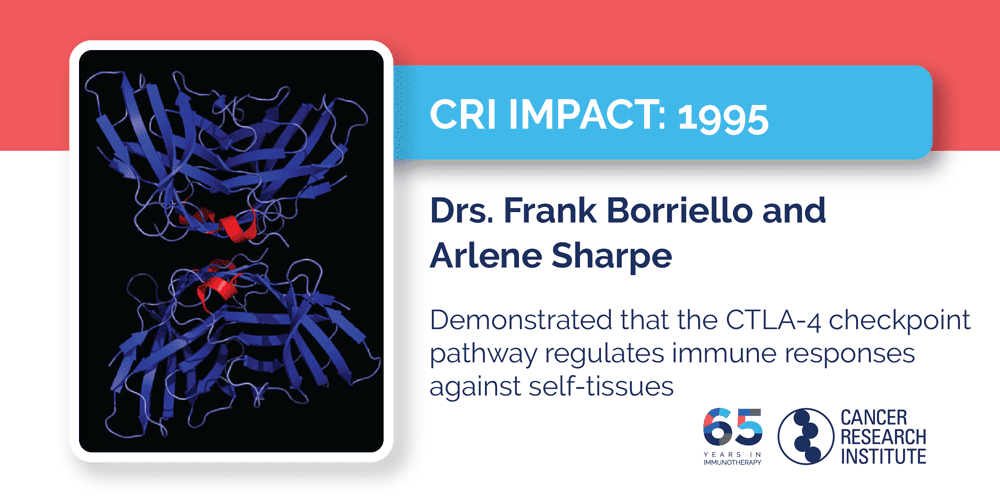
In 2011, ipilimumab became the first checkpoint immunotherapy to be approved by the FDA, after it became the first treatment of any type to improve survival in patients with advanced melanoma in a phase III clinical trial. By blocking the CTLA-4 pathway that shuts T cells off, patients’ immune systems were able to launch stronger immune responses against their tumors and, in some cases, provide them with long-term benefits and potentially even cures. Although CTLA-4 was discovered in 1987, it wasn’t until the 1990s that its role in the behavior of immune cells was determined once and for all. One of the pivotal studies that helped established our understanding of this important immune checkpoint was led by Dr. Arlene Sharpe of Harvard Medical School and Brigham and Women's Hospital who, along with Dr. Frank Borriello, a CRI-funded postdoctoral fellow in her lab, demonstrated unambiguously that CTLA-4 acted as a negative switch—a brake—on T cells. In their work, they engineered mice without CTLA-4 receptors and found that they lacked proper immune balance and were characterized by out-of-control immune responses that attacked healthy tissues and organs. This breakthrough insight helped launch the field of checkpoint immunotherapy and, after others showed that blocking CTLA-4 could enhance immune responses against cancer, led directly to the clinical development of the anti-CTLA-4 drug ipilimumab.
2006: Drs. E. John Wherry, David Masopust, and Rafi Ahmed
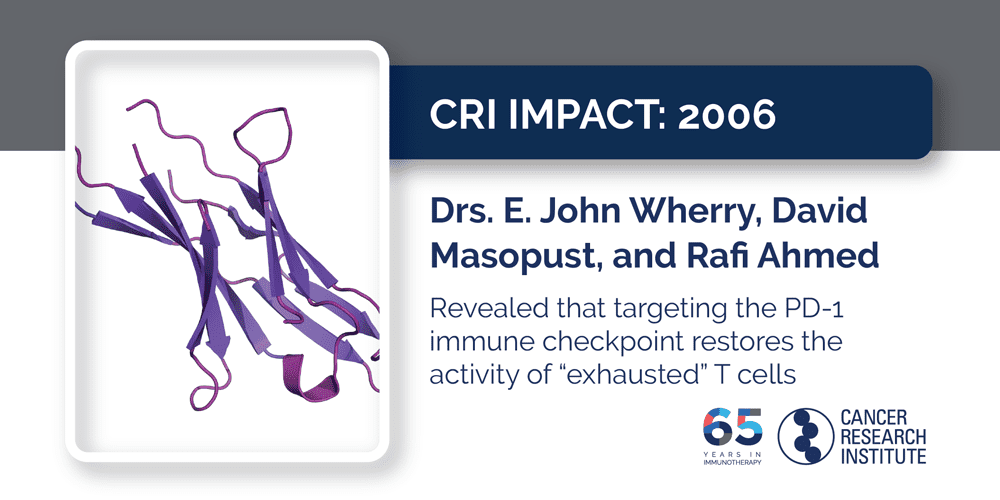
When our body detects an intruder and launches an immune response, our healthy cells express a protein on their surface called PD-L1 that can help ensure they don’t get caught in the crossfire. If any T cell engages a healthy cell, this PD-L1 binds to the PD-1 immune checkpoint on T cells, which acts as a brake that “exhausts” them so the healthy cell isn’t attacked. Unfortunately, virus-infected cells and even tumor cells can trick the immune system and protect themselves through the PD-1/PD-L1 checkpoint, too. In CRI-funded work at Emory University, Drs. E. John Wherry, David Masopust, and Rafi Ahmed showed that by blocking the activity of this PD-1 receptor, the activity of “exhausted” T cells could be restored, enhancing their ability to eliminate virus-infected cells. Subsequently, this approach was also shown to work against cancer, which then spurred the development of numerous PD-1/PD-L1 checkpoint immunotherapies, five of which are now approved to treat a variety of advanced cancers. One even became the only treatment of any type to be approved for patients regardless of their cancer type, as long as their tumors have unstable genomes characterized by high microsatellite instability (MSI-hi).
2014: Drs. Matthew Gubin and Robert Schreiber
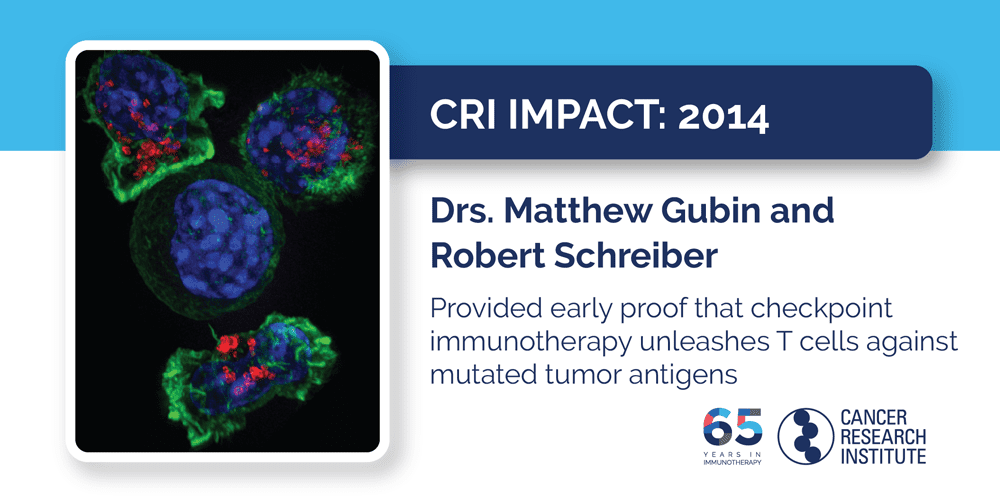
Checkpoint immunotherapies, like those that target PD-1/PD-L1 and CTLA-4 pathways mentioned above, prevent cancer-fighting T cells from being shut down and have been very effective against certain types of advanced cancer. Despite the clinical success, the specifics of how these empowered immune cells were able to eliminate tumors weren’t immediately known. As this work by Dr. Robert Schreiber and CRI postdoctoral fellow Dr. Matthew Gubin showed, these checkpoint immunotherapies enable T cells to target the abnormal tumor markers (known as neoantigens) that arise from mutations and distinguish cancer cells from normal cells. While those neoantigen-targeting T cells existed prior to treatment, these immunotherapies were able to re-activate them and enable their continued activity against tumor cells. These important insights into the mechanisms responsible for the effectiveness of these current immunotherapies also suggested strategies that could potentially improve their application and expand their benefits to more patients.
2016: Drs. Ton Schumacher, Roger Lo, and Antoni Ribas
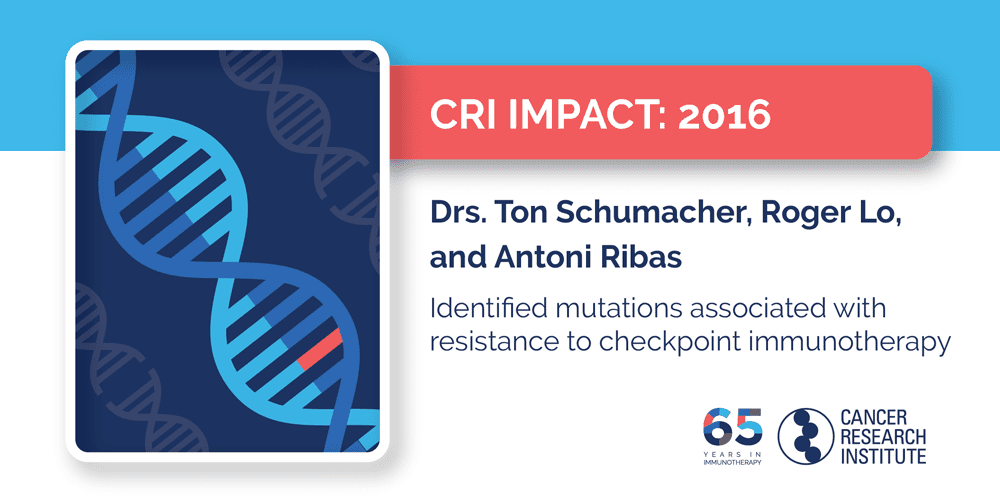
While immunotherapy has the potential to help all patients, current PD-1/PD-L1 checkpoint immunotherapies still only work in certain subsets of patients. After identifying traits that made patients more likely to respond to checkpoint immunotherapy, Dr. Antoni Ribas of the University of California, Los Angeles (UCLA),, along with fellow CRI-SU2C Dream Team member Dr. Ton Schumacher of The Netherlands Cancer Institute and CRI CLIP Investigator Dr. Roger Lo of UCLA, found certain mutations that were associated with resistance to checkpoint immunotherapy. In particular, they found that loss-of-function mutations in cancer cells’ JAK1 and JAK2 genes—crucial components in the interferon-gamma immune signaling pathway—were associated with both primary and acquired resistance to immunotherapy in patients with advanced melanoma. Meanwhile, a deleterious mutation in the gene encoding β2-microglobulin, which plays an important role in allowing cells to present antigens to the immune system through the major histocompatibility complex (MHC), was also observed in a patient who developed resistance to PD-1 checkpoint immunotherapy. The subsequent lack of surface MHC likely enabled these cancer cells to “hide” from the adaptive immune system. This knowledge of how cancer cells can potentially evade immune responses and resist immunotherapy through specific mutations has improved doctors’ understanding of these challenging cases, and pointed to promising strategies that may one day help provide relief to these patients.
2015: Drs. Stefani Spranger and Thomas Gajewski
T cells are the most powerful cancer-fighting cells in our immune system, so it should be no surprise that most immunotherapies seek, either directly or indirectly, to enable killer T cells to attack and eliminate tumors. Some, such as checkpoint inhibitors, work by empowering T cells within the tumor itself, and as a result, work best in patients whose tumors have already been recognized and infiltrated by T cells. Unfortunately, in the majority of patients, their T cells have not been able to infiltrate their tumors, which can enable cancer to evade immune responses and limit the effectiveness of checkpoint immunotherapy. In 2015, with funding from a CRI postdoctoral fellowship, Dr. Stefani Spranger worked with Dr. Thomas Gajewski at the University of Chicago to reveal one way that cancer can protect itself by preventing T cells from getting into tumors. After identifying an association between activation of the Wnt/β-catenin pathway and lack of immune infiltration in human melanoma samples, they used a mouse model of melanoma to determine that this Wnt/β-catenin pathway activity diminished dendritic cell activity and dampened recruitment of T cells to the tumor site. Consequently, checkpoint immunotherapy was less effective at eliminating these tumors that lacked T cells. Building on this insight, approaches have now been developed to target this pathway and potentially improve immunotherapy’s effectiveness in these patients.
2004: Drs. Kang Liu and Ralph Steinman
The amazing power of our immune system stems from its ability to adapt—to learn what threats “look like” and then launch precise responses against them. While T cells directly engage and eliminate cancer cells, immune cells known as dendritic cells (DCs) play an equally important role in helping to coordinate these overall responses by enabling T cells to successfully seek out the appropriate targets. In addition to recruiting T cells to the sites of tumors, by processing and then presenting a cancer cell’s markers (also known as its antigens) on their surface, dendritic cells can help educate T cells about cancer's identity. In this work, Dr. Ralph Steinman and Dr. Kang Liu, a CRI-funded postdoctoral fellow in Steinman's lab at The Rockefeller University, revealed that the CD40 pathway provides a crucial link between the “innate” and adaptive branches of the immune system and is required to promote these adaptive immune responses through the generation of “killer” T cells as well as “helper” T cells. As a result, immunotherapies designed to activate this CD40 pathway and strengthen patients’ immune responses against cancer are now being evaluated in clinical trials, including one of our CRI Clinical Accelerator clinical trials that is currently testing a CD40-based combination immunotherapy in patients with pancreatic cancer.
2017: Drs. Mohammad Rashidian and Hidde Ploegh
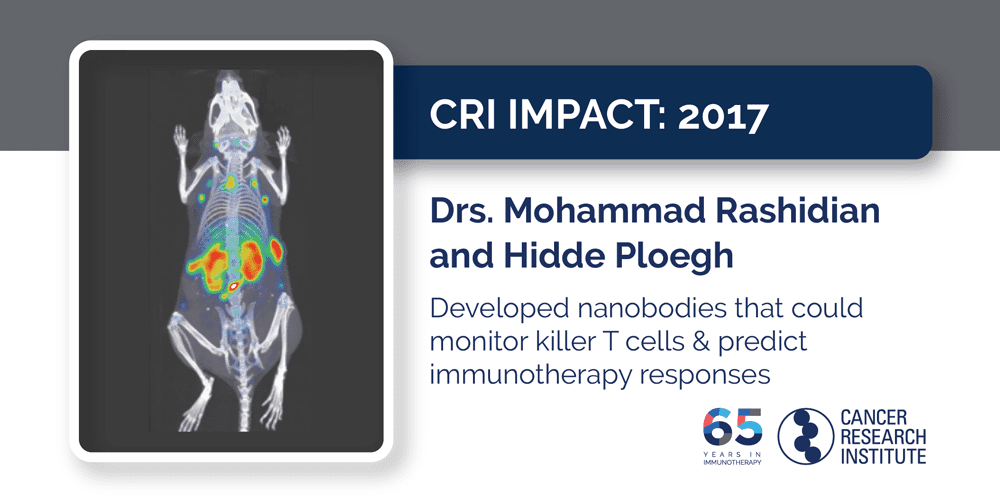
The knowledge that checkpoint immunotherapy works best against tumors that have already been recognized and targeted by “killer” T cells is incredibly valuable to doctors because it can help them identify the patients who are most likely to benefit from treatment. However, in most cases, obtaining that information requires an invasive tumor biopsy. Fortunately, Drs. Hidde Ploegh and Mohamad Rashidian, a CRI-funded postdoctoral fellow in Ploegh’s lab at Harvard Medical School and Boston Children’s Hospital, showed that immuno-PET imaging could be used to visualize these killer T cells within tumors in mice. Furthermore, they also discovered that different patterns of immune infiltration into tumors were associated with different outcomes: mice whose tumors were uniformly infiltrated by T cells were much more likely to respond to checkpoint immunotherapy than those with tumors that only had pockets of T cell infiltration. This strategy is now in the process of being translated into the clinic, where it could help them determine the patients for whom immunotherapy might be a good option as well as monitor patients during treatment to track their immune activity and see if they are responding.
For more on cancer immunotherapy and our 30 Days of CRI Impact, be sure to follow #CIM18 on Twitter.

