Oncolytic Virus Therapy
How Oncolytic Virus Therapy is Changing Cancer Treatment
Reviewed by:
John C. Bell, PhD
Ottawa Hospital Research Institute, University of Ottawa
Oncolytic viruses are a form of immunotherapy that uses viruses to infect and destroy cancer cells.
Viruses are particles that infect or enter our cells and then use the cell’s genetic machinery to make copies of themselves and subsequently spread to surrounding uninfected cells. Infection by certain viruses has been implicated in the development of certain cancers, such as the hepatitis B virus (HBV) in liver cancer and the human papilloma virus (HPV) in cervical cancer and head and neck cancer. (In the case of HPV and hepatitis, another type of immunotherapy—cancer vaccines—has shown the ability to prevent infection and protect against the formation of these HPV- and HBV-related cancers.)
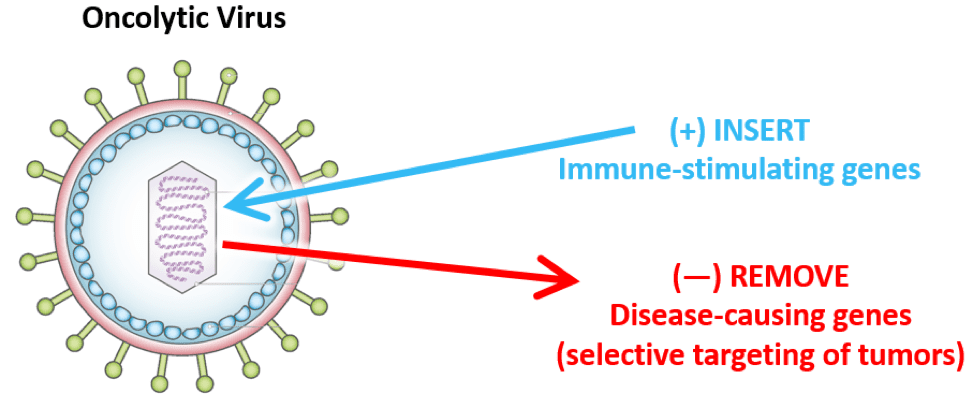
More recently, viruses have been used to target and attack tumors that have already formed. These viruses—some, but not all, of which have been modified—are known as oncolytic viruses and they represent a promising approach to treating cancer for several reasons:
- Cancer cells often have impaired antiviral defenses that make them susceptible to infection.
- These natural viruses can be engineered to give them advantageous properties, including decreasing their ability to infect healthy cells as well as granting them the ability to deliver therapeutic payloads specifically to tumors and produce immune-boosting molecules once they infect tumor cells.
- After infection, these oncolytic viruses can cause cancer cells to “burst”—killing the cancer cells and releasing cancer antigens. These antigens can then stimulate immune responses that can seek out and eliminate any remaining tumor cells nearby and potentially anywhere else in the body.
In 2015, the U.S. Food and Drug Administration (FDA) approved the first oncolytic virus immunotherapy for the treatment of cancer—T-VEC for melanoma. This treatment involves a herpes virus that has been engineered to be less likely to infect healthy cells as well as cause infected cancer cells to produce the immune-stimulating GM-CSF protein.
Oncolytic Virus Therapy Treatment Options
There is currently one oncolytic virus therapy approved by the FDA for the treatment of cancer:
- T-VEC (Imlygic®): a modified herpes simplex virus (HSV) that infects tumor cells and promotes their destruction; approved for subsets of patients with melanoma
Side Effects
Side effects may vary according to the type of oncolytic virus—and what exactly it targets—and may also be influenced by the location and type of cancer as well as a patient’s overall health. Due to their potential to infect healthy cells as well as stimulate overall immune activity, sometimes oncolytic viruses may cause the immune system to attack healthy cells, and their use may carry some risk of infection. Patients should always consult their doctors and the rest of their care team to gain a better and fuller understanding of the potential risks and side effects associated with specific oncolytic viruses.
Common side effects associated with the currently approved oncolytic virus may include but are not limited to: chills, fatigue, flu-like symptoms, injection site pain, nausea, and fever.
CRI’s Impact in Oncolytic Virus Therapy
Throughout CRI’s history, we have supported a variety of basic research projects aimed at improving our understanding of the how viruses interact with our cells and immune system. We have also supported translational and clinical efforts that seek to use these insights in the development of oncolytic virus therapies for cancer patients in the clinic.
In 2018, CRI CLIP investigator John C. Bell, PhD, of the Ottawa Hospital Research Institute, revealed that treating triple-negative breast cancer patients with oncolytic virus therapy prior to surgery increased their likelihood of responding to checkpoint immunotherapy.
Additionally, CRI is currently providing funding support for a phase I/II clinical trial (NCT02963831) combining an oncolytic virus and a checkpoint inhibitor in patients with advanced appendiceal cancer, colorectal cancer, and ovarian cancer. This trial is being led by and Kunle Odunsi, MD, PhD, of the Roswell Park Comprehensive Cancer Center, and Dmitriy Zamarin, MD, PhD, of Memorial Sloan Kettering Cancer Center.
Oncolytic Virus Clinical Trial Platforms
Oncolytic virus platforms under evaluation in clinical trials include:
- Adenovirus: a family of common viruses that can cause a wide range of typically mild effects including sore throat, fatigue, and cold-like symptoms
- Herpes simplex virus: a virus that can cause the formation of sores on or near the mouth
- Maraba virus: a virus found exclusively in insects
- Measles: a highly contagious virus that infects the respiratory tract and can cause measles
- Newcastle Disease Virus: a virus primarily found in birds; can cause mild conjunctivitis and flu-like symptoms in humans
- Picornavirus: a family of viruses that can cause a range of diseases in mammals and birds; the coxsackie virus is an example from this family that is being clinically tested
- Reovirus: a family of viruses that can affect the gastrointestinal and respiratory tracts in a range of animal species
- Vaccinia virus: the virus that was used to help vaccinate against and eliminate smallpox; rarely causes illness in humans and is associated with a rash covering the body
- Vesicular stomatitis virus: a virus that belongs to the same family as the Maraba virus; can cause flu-like symptoms in humans
In addition to these oncolytic virus platforms currently being evaluated, new platforms and approaches are constantly being developed and investigated in clinical trials. To determine if you or someone you know might be eligible for an immunotherapy clinical trial, please consult our Clinical Trial Finder service.
Find an Immunotherapy Clinical Trial
Create a profile and fill out a questionnaire to identify immunotherapy clinical trials for which you may be eligible.
Need more information? Learn more about clinical trials.