Immune to Cancer: The CRI Blog
-
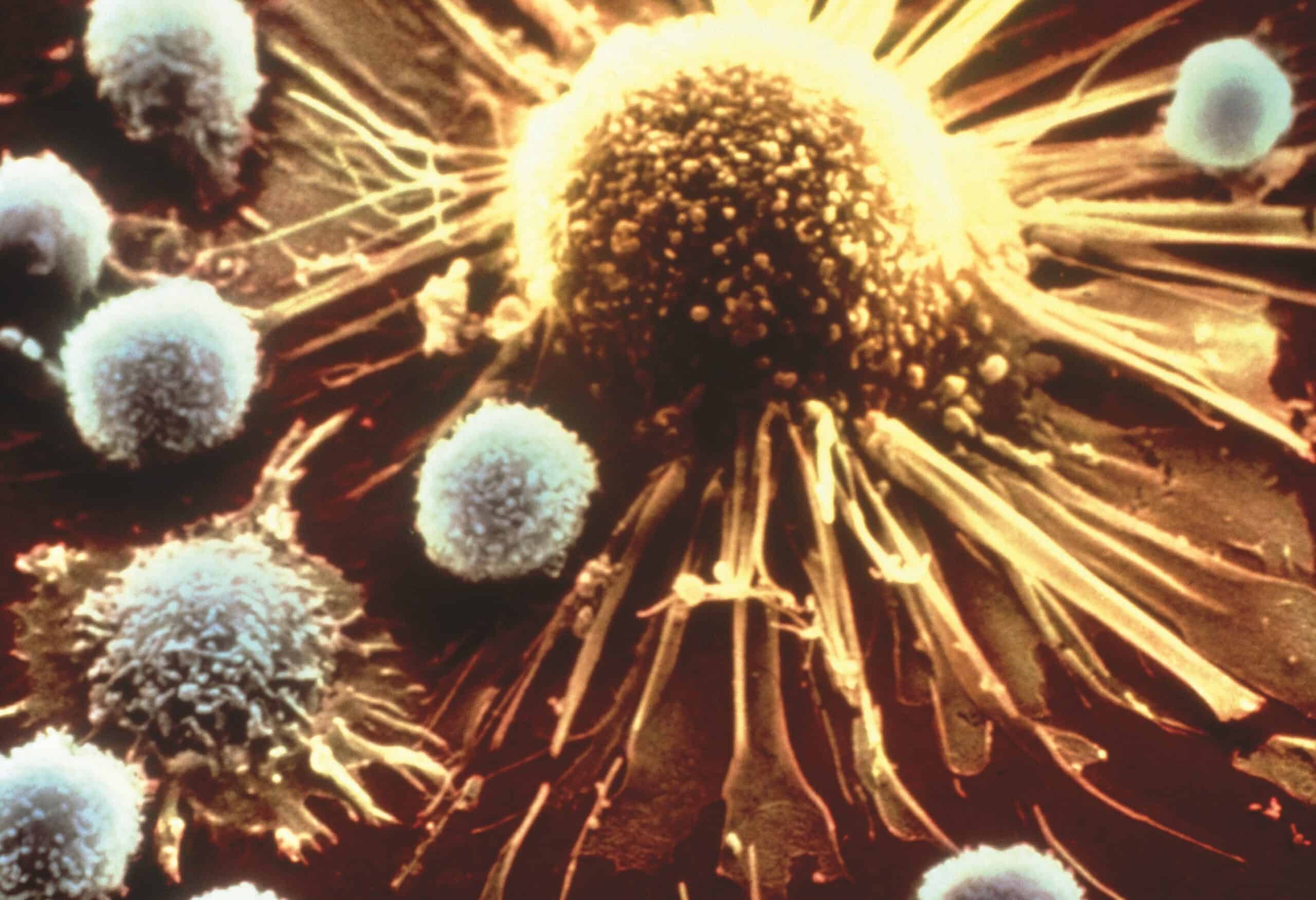
CAR T Cell Immunotherapy Approved for Adult Non-Hodgkin Lymphoma Patients
FDA approves new immunotherapy–Yescarta (axicabtagene ciloleucel)–for adult patients with relapsed or refractory non-Hodgkin large B cell lymphoma.
-
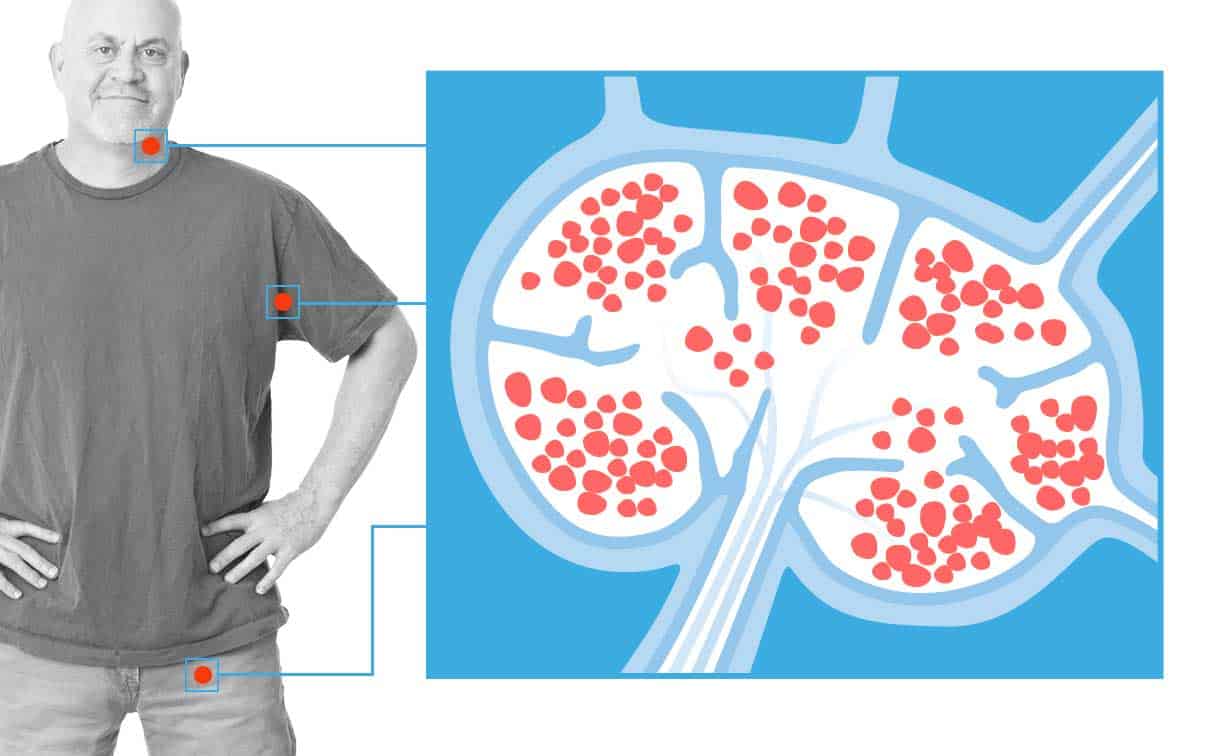
FDA Approves First-In-Class CAR T Cell Immunotherapy for Leukemia
Children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL) can now receive the CD19-targeting CAR T…
-

Recapping Our 5th Annual Cancer Immunotherapy Month
The 5th Annual Cancer Immunotherapy Month generated more than 6,000,000 impressions and raised over $500,000.
-

ASCO17 Recap: 7 Takeaways from the World’s Premier Clinical Cancer Conference
ASCO17’s presentations reveal that the field of immunotherapy is advancing along many fronts
-

CNN Highlights Cancer Immunotherapy Advances, CRI’s Pioneering Role
First-line lung cancer immunotherapy approval results from century-long effort to harness the immune system to fight cancer.
-

Cancer Research Institute Featured in New York Times Cover Story on Cancer Immunotherapy
The pioneering work of CRI and its funded scientists are profiled in this special feature from the…
-

#AACR16 Update: Vice President Biden and the National Cancer Moonshot Initiative
VP Biden addresses AACR16 seeking suggestions and solutions.
-

CRI Meets with the Vice President’s Staff on Moonshot
CRI and Vice President Biden’s Moonshot Initiative collaborate for the advancement of cancer immunotherapy research.
-

Business Insider Credits CRI with Pioneering Field of Cancer Immunotherapy
Jimmy Carter’s successful treatment with immunotherapy is thanks to CRI’s pioneering work, says Business Insider.