Immune to Cancer: The CRI Blog
-

Fourth Annual #GivingTuesday a Record Success
Here’s to you—for kicking off this season with a gift for a good reason. The Cancer Research Institute…
-
Nivolumab Becomes First FDA-Approved Checkpoint Blockade for Kidney Cancer
New hope now exists for patients with advanced kidney cancer.
-
First Monoclonal Antibody for Multiple Myeloma Receives FDA Approval
Daratumumab (Darzalex®) is the first approved immunotherapy that targets CD38—a protein found in most multiple myeloma cells.
-

CRI Feels the LOVE and Wins $250,000!
Thanks to our donors, we won second place in the Revlon LOVE IS ON Million Dollar Challenge.
-

Second Annual Immuno-Oncology 360° Conference
CRI Scientific Advisory Council member to co-chair two-day conference devoted to cancer immunotherapy.
-

Making It Count: Team CRI Adds On Miles for Cancer Research
Meet a few of the Team CRI members who ran in the 2015 TCS New York City…
-
FDA Approves Immunotherapy to Help Prevent Relapse of Melanoma
The immunotherapy drug ipilimumab (Yervoy®) can now be used to help prevent relapse after surgery.
-
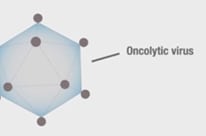
FDA Approves First in a New Class of Immunotherapies
Amgen’s oncolytic virus immunotherapy, T-VEC, gets the green light from the FDA.
-

What Is an Oncogene? Immunologists Rethink a Fundamental Cancer Concept
Immunology is reshaping how oncologists think about cancer, and even basic principles—like oncogenes—are getting a significant makeover.

Join CRI in Shaping the Future of Immunotherapy
Support the pioneering work of CRI in advancing immunotherapy.