Immune to Cancer: The CRI Blog
-
Author Shares Her Immunotherapy Experience in New Memoir
In her new memoir, Mary Elizabeth Williams documents her experience with metastatic melanoma, and her subsequent treatment…
-
Immunotherapy in the Spotlight
Dateline NBC took a closer look at this revolution known as immunotherapy and plans to tap into…
-

Atezolizumab (Tecentriq™) Receives FDA Approval for Bladder Cancer
It becomes the first FDA-approved checkpoint blockade immunotherapy for bladder cancer.
-
Cancer Research Institute Raises $1.2 Million at Annual Through the Kitchen
On Sunday, May 16, 2016, the Cancer Research Institute (CRI) held its 34th annual “Through the Kitchen”…
-

FDA Approves Nivolumab (Opdivo) for Advanced Hodgkin Lymphoma Patients
Nivolumab becomes first checkpoint immunotherapy approved for lymphoma.
-

Questions About Cancer Immunotherapy? Ask a Scientist!
We’re excited to present our newest educational initiative for patients—the Ask a Scientist video series.
-

#AACR16 Update: Vice President Biden and the National Cancer Moonshot Initiative
VP Biden addresses AACR16 seeking suggestions and solutions.
-
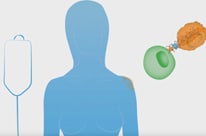
#AACR16 Update: The Promise of Synthetic Biology and CAR T Cell Technologies
Genetically engineered cells can be customized to target cancer.
-
#AACR16 Update: New Hope for Patients with Rare Skin Cancer, Head & Neck Cancer
Anti-PD-1 blockade and two other types of immunotherapy effective in treating multiple tumor types.

Join CRI in Shaping the Future of Immunotherapy
Support the pioneering work of CRI in advancing immunotherapy.