Immune to Cancer: The CRI Blog
-
CICON17 Day 4 Recap: The Tumor Microenvironment and Oncolytic Viruses
The final day of CICON17 explored two newer immunotherapy approaches that reflect broader understanding of tumor-immune interactions…
-
CICON17 Day 3 Recap: Combinations, Checkpoint Immunotherapy Resistance, Immunomodulators, and Microbiota
Day 3 of the CICON17 focused on combination therapies, immunomodulators, overcoming immune suppression, and the role bacteria in…
-
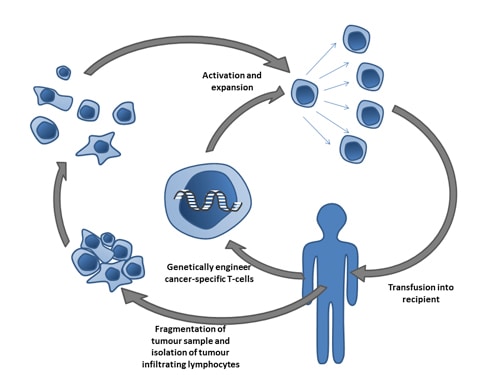
CICON17 Day 2 Recap: Biomarkers, Novel Agents, and Adoptive Cell Therapies
Day 2 of CICON17 offered a focused exploration of the latest discoveries and data from scientific studies…
-
CICON17 Day 1 Recap: Neoantigens, Vaccines, and Overcoming Immunotherapy Resistance
Day 1 of CICON17 explored how to identify and target neoantigens with vaccines and other strategies to…
-
FDA Approves First-In-Class CAR T Cell Immunotherapy for Leukemia
Children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL) can now receive the CD19-targeting CAR T…
-

Highlights of the 2nd Annual Rational Combinations 360° Conference
Biomarkers will be crucial to taking advantage of combination immunotherapy’s full potential
-
Nivolumab Approved for Patients with Chemotherapy-Resistant, Metastatic, MSI-H Colorectal Cancer
Nivolumab becomes second checkpoint immunotherapy approved for colorectal cancer
-

Recapping Our 5th Annual Cancer Immunotherapy Month
The 5th Annual Cancer Immunotherapy Month generated more than 6,000,000 impressions and raised over $500,000.
-

More Than $875,000 Raised at the 2017 Answer to Cancer Cycling Event and 5K Family Fun Run and Walk
With more than 450 bikers, runners, walkers, and volunteers, the 2017 Answer to Cancer was the best…

Join CRI in Shaping the Future of Immunotherapy
Support the pioneering work of CRI in advancing immunotherapy.