The Cancer Research Institute (CRI), a nonprofit organization dedicated to the discovery and development of powerful immunotherapies for all cancers, announced today the publication in Nature Reviews Drug Discovery of its newest analysis of the global landscape of PD-1/PD-L1 inhibitor drug development for cancer treatment. The report, titled, “Trends in clinical development of PD-1/PD-L1 inhibitors,” provides an objective look at the major trends in clinical development and regulatory approvals of PD-1/PD-L1-targeting monoclonal antibodies (mAbs), which are now the standard of care for 16 different cancer types and one tissue-agnostic indication. This latest analysis follows CRI’s first report on this topic published in September 2017 in Annals of Oncology.
“Since we conducted our first survey of the PD-1/PD-L1 mAb landscape in September 2017, activity around this type of immunotherapy has grown significantly, both in the number of agents receiving regulatory approval and the number of clinical trials worldwide,” said Jia Xin “Annie” Yu, PhD, research analyst for the CRI Anna-Maria Kellen Clinical Accelerator (CRI Clinical Accelerator) and the first author of the report. “The field is particularly focused on combination therapy, as exemplified by the doubling over the last two years in the number of trials testing PD-1/PD-L1 inhibitors in combination with other drugs.”
“With the growth seen in the number of trials, drug combinations, and cancer indications within the global PD-1/PD-L1 landscape, it is imperative that meaningful biomarkers be found for each combination strategy in order to identify the responding patients,” added Vanessa Hubbard-Lucey, PhD, director of the CRI Clinical Accelerator. “Through our Clinical Accelerator, CRI is well positioned to help academia and industry partners collaborate to develop these novel biomarkers as well as test novel combination therapies, especially those designed to help patients overcome resistance to treatment with checkpoint inhibitors.”
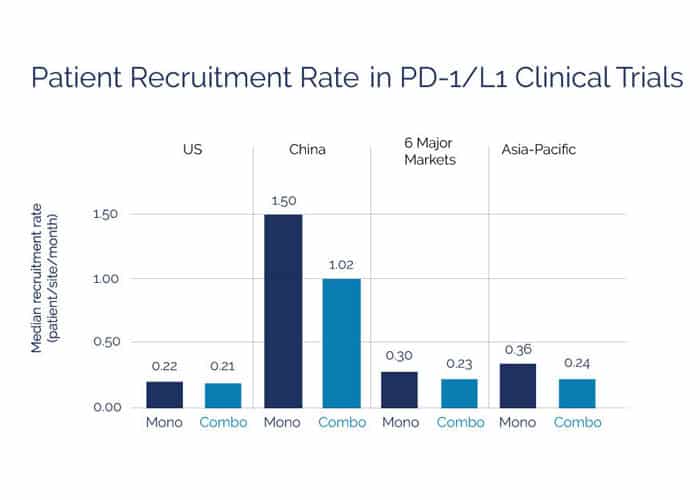
This updated analysis underscores the large number of available patients in emerging markets and how this contributes to faster trial enrollment and rapid clinical drug development within these markets. Shared data from these trials may also potentially accelerate immunotherapy development elsewhere around the world.
“The convergence of innovation across various disciplines has generated practice-changing PD-1/PD-L1 therapies for cancer patients, and we are encouraged by the continued growth in this field,” said Jill O’Donnell-Tormey, PhD, chief executive officer and director of scientific affairs at the Cancer Research Institute. “As investigational monotherapies and combination therapies in development will need to be evaluated in clinical trials, CRI is committed to funding research that would make this possible.”
To access an interactive dashboard of the report, visit the CRI website at cancerresearch.org/pd1l1-landscape.
Reference: Yu JX, Hodge JP, Oliva C, Neftelinov ST, Hubbard-Lucey VM, Tang, J. Trends in clinical development of PD-1/PD-L1 inhibitors. Nat Rev. Drug. Discov.https://www.nature.com/articles/d41573-019-00182-w
About the Cancer Research Institute
The Cancer Research Institute (CRI), established in 1953, is the world’s leading nonprofit organization dedicated exclusively to saving more lives by fueling the discovery and development of powerful immunotherapies for all types of cancer. Guided by a world-renowned Scientific Advisory Council that includes four Nobel laureates and 24 members of the National Academy of Sciences, CRI has invested $420 million in support of research conducted by immunologists and tumor immunologists at the world’s leading medical centers and universities, and has contributed to many of the key scientific advances that demonstrate the potential for immunotherapy to change the face of cancer treatment. To learn more, go to cancerresearch.org.
About the CRI Anna-Maria Kellen Clinical Accelerator
CRI’s clinical program, the Anna-Maria Kellen Clinical Accelerator, is a unique academia-nonprofit-industry collaboration model that serves as an “incubator” that delivers multi-center clinical trials for promising new immunotherapy combinations. CRI’s venture philanthropy fund supports clinical trials within this program, which fosters a collaborative environment that enables scientists to advance their most ambitious research ideas and accelerates studies that one group or company could not do alone. To learn more about the Anna-Maria Kellen Clinical Accelerator, go to cancerresearch.org/clinical-accelerator.
Media Contact:
Brian Brewer
Director of Marketing and Communications
Phone: 212-688-7515 ext. 242
Fax: 212-832-9376
Email: [email protected]