The Parker Institute for Cancer Immunotherapy (PICI) and the Cancer Research Institute (CRI) announced today the dosing of the first patients in the REVOLUTION trial, a phase I, open label platform study of novel immunotherapy combinations for first-line treatment of metastatic pancreatic cancer.
“PICI is built on the promise of bringing together the brightest minds to come up with innovative solutions to cancer’s toughest problems, and this trial is a direct result,” said Ute Dugan, MD, PhD, PICI’s Chief Medical Officer. “The innovative design also represents a new model of collaborative, end-to-end drug development that allows us to more rapidly advance promising therapeutic combinations from bench to bedside to market, and then to patients.”
REVOLUTION uses a platform trial design that allows for more rapid testing of several therapies with more adaptability than a standard clinical trial. In a traditional clinical trial, all elements of the study must be approved prior to beginning. When the study meets its endpoint, researchers must decide what study to do next based on the findings.
In contrast, a platform design allows researchers to interpret the data during the trial and to make changes, such as testing additional treatments, expanding the number of people receiving a therapy that shows promise, or discontinuing a therapy that does not look promising. This allows investigators to follow the science with flexibility and efficiency, avoiding some of the administrative and logistical hurdles involved in starting up individual trials in a safe way and decreasing the likelihood that patients will receive ineffective treatments.
“Platform studies offer a science-informed flexibility that is better able to keep pace with the accelerating advances in clinical cancer immunology, especially those that involve promising drug combinations like those brought together through the Cancer Research Institute’s collaborations with PICI and our academic, industry, and other nonprofit partners,” said Jill O’Donnell-Tormey, PhD, CEO and Director of Scientific Affairs at CRI.
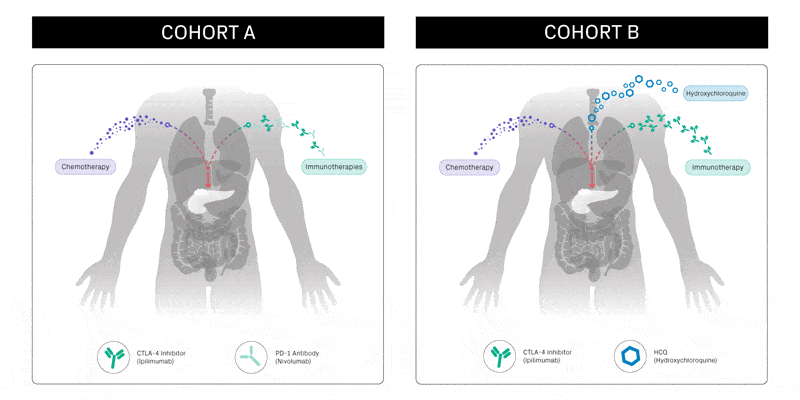
REVOLUTION currently consists of two groups of patients. Cohort A will test a combination of standard-of-care chemotherapy, the CTLA-4 inhibitor ipilimumab and the PD-1 inhibitor nivolumab. In addition to providing valuable information on the efficacy of the combination itself, this cohort builds on the findings from PICI’s and CRI’s PRINCE trial, which suggested that the combination of chemotherapy and anti-PD-1 treatment may improve survival compared with chemotherapy alone.
Cohort B will investigate the combination of chemotherapy, ipilimumab and hydroxychloroquine (HCQ). HCQ is a drug that can stop a process called autophagy, which cells use to recycle their own materials to survive, particularly under stress. Previous research has indicated autophagy is a key mechanism by which metastatic pancreatic cancer cells evade the immune system.
The first patients have been dosed in each cohort.
This trial will also further explore biomarker data generated by the PRINCE study, aiming to better understand which patients stand to benefit from immunotherapy.
“This is an exciting next step in PICI’s and CRI’s commitment to deliver bold, science-driven ideas, and this trial shows how those efforts build on each other as the research progresses,” said PICI researcher and CRI Scientific Advisory Council member Robert H. Vonderheide, MD, DPhil, director of the Abramson Cancer Center of the University of Pennsylvania and the study’s overall principal investigator. “REVOLUTION will make use of the same capabilities as PRINCE while adding a framework that can test and advance potentially promising combination therapies more efficiently than standard clinical trials.”
REVOLUTION is also designed with the greater field in mind, building off PICI’s and CRI’s shared goals of breaking silos and creating efficiencies through collaboration. PICI has partnered with the Pancreatic Cancer Action Network (PanCAN) and its Precision PromiseSM phase 2/3 platform study in metastatic pancreatic cancer for exactly this purpose. If a cohort of REVOLUTION proves successful, it can be considered for further development under the Precision Promise umbrella. Once again, this accelerates the clinical trial process, ensuring potentially lifesaving therapies make their way to patients as quickly as possible. This partnership also advances CRI’s commitment to discovering and developing immune-based treatments that improve outcomes for all cancer patients, including those who face pancreatic and other hard-to-treat cancers.
In addition to Penn, sites on the trial include the Dana-Farber Cancer Institute, Memorial Sloan Kettering Cancer Center, the University of California, San Francisco, the University of California, Los Angeles, the University of Texas MD Anderson Cancer Center, and Stanford Medicine.
Additional partners on the trial include the 1440 Foundation, Bristol Myers Squibb and PanCAN.
About Pancreatic Cancer
Pancreatic cancer is one of the deadliest types of tumors, and the number of diagnosed cases continues to rise each year. The disease is difficult to catch early, meaning by the time most people are diagnosed, their cancer is advanced and may have already spread. In addition, the tumors usually contain a variety of mutations, meaning one targeted therapy isn’t enough to stop the disease by itself. For patients who are diagnosed after cancer has spread to other parts of the body – a distinction that applies to more than half of all pancreatic patients – the 5-year survival rate is just three percent.
About the Parker Institute for Cancer Immunotherapy
The Parker Institute for Cancer Immunotherapy (PICI) is radically changing the way cancer research is done. Founded in 2016 through a $250 million gift from Silicon Valley entrepreneur and philanthropist Sean Parker, the San Francisco-based nonprofit is an unprecedented collaboration between the country’s leading immunotherapy researchers and cancer centers, including Memorial Sloan Kettering Cancer Center, Stanford Medicine, the University of California, Los Angeles, the University of California, San Francisco, the University of Pennsylvania and The University of Texas MD Anderson Cancer Center. The institute also supports top researchers at other institutions, including City of Hope, Dana-Farber Cancer Institute, Fred Hutchinson Cancer Research Center, Icahn School of Medicine at Mount Sinai, Institute for Systems Biology and Washington University School of Medicine in St. Louis. By forging alliances with academic, industry and nonprofit partners, PICI makes big bets on bold research to fulfill its mission: to accelerate the development of breakthrough immune therapies to turn all cancers into curable diseases. Find out more at www.parkerici.org.
About the Cancer Research Institute
The Cancer Research Institute (CRI), established in 1953, is a top‐rated U.S. nonprofit organization dedicated exclusively to saving more lives by fueling the discovery and development of powerful immunotherapies for all cancers. Guided by a world‐renowned Scientific Advisory Council that includes four Nobel laureates and 27 members of the National Academy of Sciences, CRI has invested $474 million in support of research conducted by immunologists and tumor immunologists at the world’s leading medical centers and universities and has contributed to many of the key scientific advances that demonstrate the potential for immunotherapy to change the face of cancer treatment. To learn more, go to cancerresearch.org.
Contact Information
John Infanti
Senior Science Communications Manager
Parker Institute for Cancer Immunotherapy
[email protected], +1-628-899-7604
Brian M. Brewer
Chief Communications Officer
Cancer Research Institute
[email protected], +1-212-688-7515 x242
